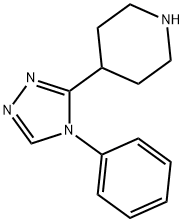
4-(4-Phenyl-4H-1,2,4-triazol-3-yl)piperidine synthesis
- Product Name:4-(4-Phenyl-4H-1,2,4-triazol-3-yl)piperidine
- CAS Number:94225-92-0
- Molecular formula:C13H16N4
- Molecular Weight:228.29
887-82-1
0 suppliers
inquiry

94225-92-0
6 suppliers
inquiry
Yield:94225-92-0 8.67 g (94.7%)
Reaction Conditions:
in sodium hydroxide;chloroform;acetic acid;
Steps:
12.C 4-(4-Phenyl-4H-1,2,4-triazol-3-yl)-piperidine
PART C 4-(4-Phenyl-4H-1,2,4-triazol-3-yl)-piperidine The starting material from Part B (8.92 g, 40.1 mmol) dissolved in 80 ml acetic acid is treated with 1.6 g of platinum oxide and hydrogenated in a Parr Bomb at 51.5 psi initial pressure. The pressure drops to 39.5 psi after 64 hours, but the reaction is continued for an additional 4 days (final pressure, 39.0 psi). The crude reaction mixture, including catalyst, is quenched in cold aqueous sodium hydroxide, treated with chloroform, and both layers are filtered through celite to remove platinum. The organic layer is separated, dried over sodium sulfate, and concentrated in vacuo to 10.79 g of solid which is crystallized from methanol/ethyl acetate mixtures (after Darco decolorizing carbon treatment) to afford 8.67 g (94.7%) of needles, in two crops, metling point 165°-169° C.: IR (nujol) 3291 cm-1; UV (95% ethanol) 256 nm, 257 nm with sh at 261, 264, 280, and 310 nm; NMR (CDCl3) 8.15, 7.2- 7.6, 1.7-3.2; mass spectrum shows a weak molecular ion peak at m/e 228 with a strong fragment ion at m/e 172. Anal. Calcd. for C13 H16 N4, mw 228.30: C, 68.39; H, 7.07; N, 24.55. Found: C, 68.08; H, 7.02; N, 24.10. An undried sample analyzed correctly for a 1/2 methanol solvate.
References:
US4481360,1984,A