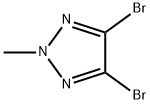
4,5-DibroMo-2-Methyl-2H-1,2,3-triazole synthesis
- Product Name:4,5-DibroMo-2-Methyl-2H-1,2,3-triazole
- CAS Number:28938-17-2
- Molecular formula:C3H3Br2N3
- Molecular Weight:240.88
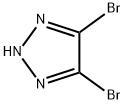
22300-52-3

74-88-4

28938-17-2
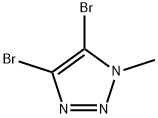
25537-64-8
10.0 g (44.1 mmol) of 4,5-dibromo-2H-1,2,3-triazole (commercially available) was dissolved in 90 mL of tetrahydrofuran, 6.1 g (44.2 mmol) of potassium carbonate was added as a base, and the reaction mixture was cooled to -10 °C. Subsequently, 7.5 g (53 mmol) of iodomethane was slowly added. The reaction system was warmed to 35-40 °C with continuous stirring until the reaction was complete. Upon completion of the reaction, the reaction was quenched by the addition of 50 mL of water and the tetrahydrofuran (~90 mL) was removed by distillation. Extraction was carried out with methyl tert-butyl ether and the organic phase was dried with anhydrous magnesium sulfate. Concentrated to dryness under reduced pressure, 10 mL of methyl tert-butyl ether was added to the residual solid, and 70 mL of hexane was added slowly and dropwise to induce precipitation of the solid. After addition, stirring was continued for 1 to 2 hours at room temperature. The solid was collected by filtration to give 5.8 g of pure 4,5-dibromo-1-methyl-1H-1,2,3-triazole in 57% yield.
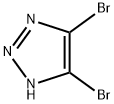
15294-81-2
129 suppliers
$6.00/250mg

74-88-4
357 suppliers
$15.00/10g

28938-17-2
66 suppliers
$20.00/100mg
Yield: 61.2%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 0 - 20;
Steps:
451.1 Step 1 :
To a solution of 4,5-dibromo-1H-1,2,3-triazole (0.401 g, 1.768 mmol) in DMF (6 mL) at 0 °C (in an ice-water bath) was added first potassium carbonate (0.366 g, 2.65 mmol) and then iodomethane (0.116 mL, 1.856 mmol) was added dropwise. After stirring lh, the reaction appears to be incomplete by HPLC. Added additional 50uL iodomethane, continued stirring, now at room temp. Reaction still appears to incomplete. Added additional 50uL iodomethane. Quenched with lOmL water. Extracted 2x 50mL EtOAc. Washed combined EtOac lx 10% LiCl, lx brine. Then dried over sodium suflate, filtered and concentrated. Loaded onto a 40g ISCO column for purification by flash chromatography, eluting with 0-100% EtOAc in hexanes. Two peaks elute, the first eluting being larger by UV absorbance, but giving no MS signal. This is 4,5-dibromo-2-methyl-2H-1,2,3-triazole (0.266 g, 1.082 mmol, 61.2 % yield), designated Isomer 2. HPLC IR 0.90 min (analytical HPLC Method A). NMR (400MHz, chloroform-d) d 4.17 (s, 3H) . The second peak is smaller by UV, but gives the correct mass and dibromo isotopic pattern in MS. This material is 4,5-dibromo-2-methyl-2H-1,2,3-triazole (0.101 g, 1.082 mmol, 61.2 % yield) designated Isomer 1. LCMS m/z 242.0 / 244.0 (M+H)+; HPLC /R 0.67 min (analytical HPLC Method A). 'H NMR (400MHz, chloroform-d) d 4.08 (s, 3H)
References:
BRISTOL-MYERS SQUIBB COMPANY;SPERGEL, Steven H.;PITTS, William J.;MERTZMAN, Michael E.;MOSLIN, Ryan M.;SHERWOOD, Trevor C.;GILMORE, John L.;DYCKMAN, Alaric J. WO2020/92196, 2020, A1 Location in patent:Page/Page column 208-209

15294-81-2
129 suppliers
$6.00/250mg

74-88-4
357 suppliers
$15.00/10g

28938-17-2
66 suppliers
$20.00/100mg

25537-64-8
54 suppliers
$35.00/100mg

22300-52-3
83 suppliers
inquiry

74-88-4
357 suppliers
$15.00/10g

28938-17-2
66 suppliers
$20.00/100mg

25537-64-8
54 suppliers
$35.00/100mg

22300-52-3
83 suppliers
inquiry

74-88-4
357 suppliers
$15.00/10g

28938-17-2
66 suppliers
$20.00/100mg

25537-64-8
54 suppliers
$35.00/100mg

28938-17-2
66 suppliers
$20.00/100mg