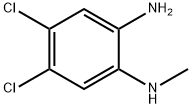
4,5-dichloro-2-(methylamino)aniline synthesis
- Product Name:4,5-dichloro-2-(methylamino)aniline
- CAS Number:42450-33-9
- Molecular formula:C7H8Cl2N2
- Molecular Weight:191.06
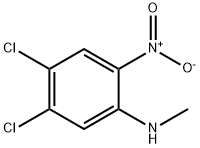
107342-18-7
9 suppliers
inquiry

42450-33-9
8 suppliers
inquiry
Yield:42450-33-9 97%
Reaction Conditions:
with iron(0);glacial acetic acid in ethanol at 60; for 2 h;
Steps:
3.1.11. Synthesis of 4,5-dichloro-N1-methylbenzene-1,2-diamine (4e)
To a solution of 4,5-dichloro-2-nitroaniline (30.0 g, 0.14 mol, 1.0 equiv.) in DMF (150 mL), 60%sodium hydride in mineral oil (6.4 g, 0.16 mol, 1.1 equiv.) was added. After 30 min of stirring in roomtemperature, methyl iodide (13.5 mL, 0.22 mol, 1.5 equiv.) was added to the reaction mixture and thesolution warmed up spontaneously. After 1h, the reaction mixture was poured into water (500 mL)and the product was extracted with ethyl acetate (3 × 100 mL). The organic phase was washed withbrine (1 × 50 mL) and dried over anhydrous magnesium sulfate. The drying agent was filtered off,the resulting filtrate was concentrated under reduced pressure and left in the fridge forcrystallization. The orange crystals of 4,5-dichloro-N-methyl-2-nitroaniline were formed, which werewashed with a small amount of ethyl acetate and dried. Yield: 25.0 g (78%). A slurry of 4,5-dichloro-N-methyl-2-nitroaniline (15.0 g, 0.07 mol, 1.0 equiv.) and iron powder (19.0 g, 0.34 mol, 5.0 equiv.) ina mixture of 95% ethanol (200 mL) and glacial acetic acid (100 mL) was stirred at 60 °C for 2h. Theprogress of reaction was monitored by TLC, when the reaction was completed; the excess of volatileswas evaporated under reduced pressure. The obtained residue was treated with water and the pHwas adjusted to ca. 8 using sodium hydroxide. The crude product was extracted with ethyl acetate (3× 150 mL), organic phase was washed with brine (1 × 50 mL) and dried over anhydrous magnesiumsulfate. Evaporation of the solvent resulted in the 4,5-dichloro-N1-methylbenzene-1,2-diamine (4e),obtained as a brown solid (12.6 g, 97% yield) which was used in the next step without furtherpurification. M.p. 100.0-101.0 °C, R.f. = 0.35 (hexane:ethyl acetate 9:1 v/v). 1H NMR (500 MHz, CDCl3)δ 6.72 (s, 1H, HAr), 6.62 (s, 1H, HAr), 3.30 (bs, 3H, NH2+NH), 2.81 (s, 3H, Me); 13C NMR (125 MHz,CDCl3) δ 138.8, 132.8, 123.2, 120.3, 117.1, 112.0, 31.0; HRMS (ESI): m/z [M + H]+ calcd for C7H9Cl2N2:191.01373, 193.01078, 175.00785, found: 191.01403, 193.01110, 175.00788;
References:
Bieszczad, Bartosz;Dudek, Marta K.;Garbicz, Damian;Grzesiuk, El?bieta;Mieczkowski, Adam;Trzybiński, Damian;Wo?niak, Krzysztof [Molecules,2020,vol. 25,# 12]
2339-78-8
162 suppliers
$9.00/1g

42450-33-9
8 suppliers
inquiry
6641-64-1
224 suppliers
$6.00/5g

42450-33-9
8 suppliers
inquiry