(4,5-DIHYDRO-THIAZOL-2-YLSULFANYL)-ACETIC ACID synthesis
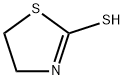
- Product Name:(4,5-DIHYDRO-THIAZOL-2-YLSULFANYL)-ACETIC ACID
- CAS Number:5685-15-4
- Molecular formula:C5H7NO2S2
- Molecular Weight:177.24
Yield:5685-15-4 91%
Reaction Conditions:
with triethylamine in dichloromethane at 20; for 72 h;
Steps:
4.2.1. 2-((4,5-Dihydrothiazol-2-yl)thio)acetic acid (5)
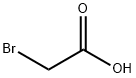
To a solution of thiazolidine-2-thione (4) (8.4 mmol) in CH2Cl2and bromoacetic acid (8.4 mmol) was added triethylamine and the mixture was stirred a room temperature for 72 h. Then, the resulting triethylammonium bromide was filtered off. In order toremove dissolved salts the, the solution was also passed through asilica gel [Merck 60 (400-230 mesh)] column using ethyl acetate aseluent. The solvent was evaporated to dryness to give a white solid(91%). Mp 125e126 C. FTIR (KBr) nmax 3002, 2957, 2786, 2365,1885,1710, 1557, 1388, 1317, 1186, 1036, 895, 678, 656, 447 cm1. 1H NMR(500 MHz, CDCl3, 25 C) d 4.25 (t, 2H, J 8.0 Hz), 3.71 (s, 2H), 3.56 (t,2H, J 8.0 Hz) ppm. 13C NMR (125 MHz, CDCl3, 25 C) d 173.6, 169.1,61.8, 36.5, 35.4 ppm. Anal. Calcd for C5H7NO2S2: C, 33.90; H, 3.95; N,7.91; S, 36.16. Found: C, 34.11; H, 4.12; N, 7.83; S, 36.26.
References:
de la Concepción, Juan García;ávalos, Martín;Babiano, Reyes;Cintas, Pedro;Jiménez, José L.;Light, Mark E.;Palacios, Juan C. [Tetrahedron,2017,vol. 73,# 12,p. 1551 - 1560]