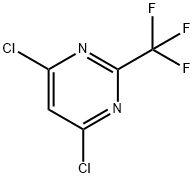
4,6-dichloro-2-(trifluoromethyl)pyrimidine synthesis
- Product Name:4,6-dichloro-2-(trifluoromethyl)pyrimidine
- CAS Number:705-24-8
- Molecular formula:C5HCl2F3N2
- Molecular Weight:216.98
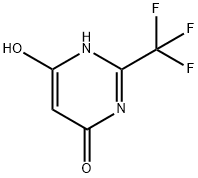
672-47-9

705-24-8
Example 2: Synthesis of 4,6-dichloro-2-trifluoromethylpyrimidine (Compound V) Step 2: In a 5L round-bottomed flask equipped with a mechanical stirrer, 4,6-dihydroxy-2-trifluoromethylpyrimidine (VI; 540 g; 3.0 mol) was mixed with phosphorus trichloride (1300 mL; 2147 g; 14.0 mol). Triethylamine (607 g; 6.0 mol) was added slowly over a period of 1 h. Exothermic phenomena were observed during the reaction. Subsequently, the reaction mixture was heated on a dry bath for 3 hours. Upon completion of the reaction, the mixture was cooled to 40°C and then slowly poured into a mixture consisting of 10 kg of ice and 2 kg of water under continuous stirring. The mixture was extracted using dichloromethane (4 x 0.5 L). The organic phases were combined, dried with anhydrous magnesium sulfate and filtered. The filtrate was concentrated under vacuum to give the crude product V. For further purification, the purified product in the form of a colorless liquid was obtained by distillation (steam bath, 48°-50°C/2 mmHg) in 78.8% yield. Elemental Analysis Results: Calculated values (C5HCl2F3N2) : C, 27.68; H, 0.47; N, 12.91. Measured values: C, 27.45; H, 0.39; N, 12.86.

672-47-9
77 suppliers
$45.00/50mg

705-24-8
112 suppliers
$26.60/250mg
Yield: 78.8%
Reaction Conditions:
with trichlorophosphate in water;triethylamine
Steps:
2 4,6-Dichloro-2-trifluoromethylpyrimidine, Compound V, Step 2
EXAMPLE 2 4,6-Dichloro-2-trifluoromethylpyrimidine, Compound V, Step 2 4,6-Dihydroxy-2-trifluoromethylpyrimidine (VI; 540 g; 3.0 M) was stirred with phosphorous oxychloride (1300 mL; 2147 g; 14.0 M) in a 5 L round-bottom flask with mechanical stirrer and triethylamine (607 g; 6.0 M) was added over 1 hr. After the exotherm, the reaction was heated on a stem-bath for 3 hr. The reaction mixture was cooled to 40°, and then it was poured with stirring into a mixture of 10 kg ice and 2 kg water. The mixture was extracted with 4*0.5 L methylene chloride. The combined extracts were dried (MgSO4) and filtered. The filtrate was concentrated in vacuo to give the crude compound V. When further purification was desired, the crude V was distilled at 48°-50°/2 mm using a steam-bath (Lit. b.p. 38°/1 mm) to give a colorless liquid. The purified yield was 78.8%. Anal. Calcd. for C5 HCl2 F3 N2: C, 27.68; H, 0.47; N, 12.91. Found: C, 27.45; H, 0.39; N, 12.86.
References:
Bristol-Myers Squibb Co. US4963678, 1990, A