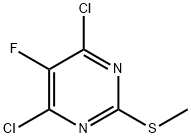
4,6-dichloro-5-fluoro-2-(methylsulfanyl)pyrimidine synthesis
- Product Name:4,6-dichloro-5-fluoro-2-(methylsulfanyl)pyrimidine
- CAS Number:6693-07-8
- Molecular formula:C5H3Cl2FN2S
- Molecular Weight:213.06
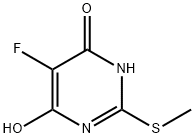
4996-41-2
7 suppliers
inquiry

6693-07-8
15 suppliers
inquiry
Yield:6693-07-8 89%
Reaction Conditions:
with trichlorophosphate at 115; for 3 h;
Steps:
E.B
Part B: 4,6-Dichloro-5-fluoro-2-(methylthio)pyrimidine. A mixture of 5-fluoro-6-hydroxy-2-(methylthio)-4(1H)-pyrimidinone (35.7 g, 0.20 mol) inPOCI3 (150 mL) was heated at 1 15 0C for 3 h. After cooling to room temperature, the reaction mixture was slowly poured into an ice-water mixture (1500 mL) and stirred for20 min. The product was extracted into ethyl acetate (3 x 800 mL), and the combined organic extracts were washed with water (2 x 1000 mL), brine (1000 mL), and dried(Na2SO4). Evaporation of the solvent provided 4,6-dichloro-5-fluoro-2-(methylthio)pyrimidine as a pale yellow solid (37.8 g, 89%). LCMS: (M+H)+: not detected.
References:
WO2009/61879,2009,A1 Location in patent:Page/Page column 63

4996-41-2
7 suppliers
inquiry

6693-07-8
15 suppliers
inquiry