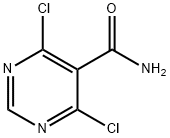
4,6-DICHLORO-5-PYRIMIDINECARBOXAMIDE synthesis
- Product Name:4,6-DICHLORO-5-PYRIMIDINECARBOXAMIDE
- CAS Number:911461-47-7
- Molecular formula:C5H3Cl2N3O
- Molecular Weight:192
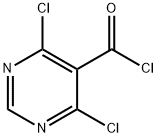
87600-97-3

911461-47-7
4,6-Dichloropyrimidine-5-carbonyl chloride (36 g, 170 mmol) was dissolved in tetrahydrofuran (200 mL) under ammonia atmosphere and the reaction was stirred at room temperature. The progress of the reaction was monitored by thin layer chromatography (TLC) until the complete disappearance of the raw material. Upon completion of the reaction, the reaction mixture was concentrated under reduced pressure and the resulting residue was partitioned with ethyl acetate (300 mL) and water (100 mL). The aqueous phase was further extracted with ethyl acetate (100 mL x 2). All organic phases were combined, washed with saturated saline (100 mL), dried over anhydrous sodium sulfate and subsequently concentrated under reduced pressure to give 4,6-dichloropyrimidine-5-carboxamide (27.2 g, yield: 83.2%) as a yellow solid. Mass spectrum (ESI, positive ion mode) m/z: 191.9 [M + H]+; 1H NMR (600 MHz, CDCl3) δ (ppm): 8.85 (s, 1H), 6.23 (br.s, 1H), 5.96 (br.s, 1H).

87600-97-3
17 suppliers
$398.75/1g

911461-47-7
21 suppliers
inquiry
Yield:911461-47-7 83.2%
Reaction Conditions:
with ammonia in tetrahydrofuran at 20;
Steps:
24.2 Step 2) 4,6-dichloropyrimidine-5-carboxamide
A solution of 4,6-dichloropyrimidine-5-carbonyl chloride (36 g, 170 mmol) in THF (200 mL) under NH3 gas atmosphere was stirred at rt. The reaction was monitored by TLC until the stating material disappeared. The reaction mixture was concentrated in vacuo and the residue was partitioned between EtOAc (300 mL) and H2O (100 mL). The aqueous phase was extracted with EtOAc (100 mL×2). The combined organic phase was washed with brine (100 mL), dried over anhydrous Na2SO4 and concentrated in vacuo to give the title compound as a yellow solid (27.2 g, yield: 83.2%). [0683] MS (ESI, pos. ion) mz: 191.9 [M+H]+; [0684] 1H NMR (600 MHz, CDCl3) δ (ppm): 8.85 (s, 1H), 6.23 (br. s, 1H), 5.96 (br. s, 1H).
References:
US2015/87658,2015,A1 Location in patent:Paragraph 0682; 0683; 0684
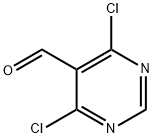
5305-40-8
325 suppliers
$10.00/1g

911461-47-7
21 suppliers
inquiry