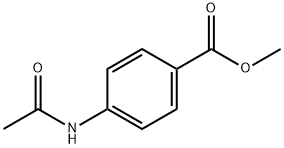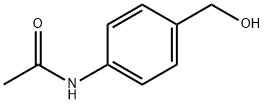
4-ACETAMIDOBENZYL ALCOHOL synthesis
- Product Name:4-ACETAMIDOBENZYL ALCOHOL
- CAS Number:16375-88-5
- Molecular formula:C9H11NO2
- Molecular Weight:165.19
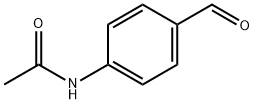
122-85-0

16375-88-5
4-Acetamidobenzaldehyde (10 g, 61.3 mmol) was dissolved in methanol (100 mL) at room temperature, followed by batchwise addition of sodium borohydride (800 mg). The reaction mixture was stirred overnight at room temperature and the reaction progress was monitored by thin layer chromatography (TLC) using 4:1 hexane:ethyl acetate as eluent. Completion of the reduction reaction was indicated when TLC showed complete disappearance of the feedstock. Subsequently, the reaction mixture was concentrated using a rotary evaporator. The concentrated residue was partitioned between water (25 mL) and ethyl acetate (4 x 50 mL), and the organic layer was separated and washed with brine (25 mL). The organic layer was dried over anhydrous sodium sulfate and the solvent was removed under reduced pressure to give the light yellow solid product N-[4-(hydroxymethyl)phenyl]acetamide. The product was dried under high vacuum to give a final 8.6 g (85% yield). The structure of the product was confirmed by 1H NMR (DMSO-d6): δ 2.0 (s, 3H), 4.5 (d, 2H), 5.2 (t, 1H), 7.25 (d, 2H), 7.55 (d, 2H), 9.95 (s, 1H).
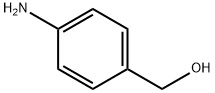
623-04-1
366 suppliers
$6.00/1g

108-24-7
0 suppliers
$14.00/250ML

16375-88-5
111 suppliers
$22.00/1g
Yield: 98%
Reaction Conditions:
with Co3O4 nanoparticles at 20; for 0.2 h;Green chemistry;chemoselective reaction;
Steps:
General procedure for acetylation of amines
General procedure: In a round-bottomed flask (25 mL) equipped with a magnetic stirrer, a mixture of aniline (0.093 g, 1 mmol) and Co3O4 (0.006 g) was prepared. Acetic anhydride (0.102, 1 mmol) was then added to the reaction mixture and stirring was continued at room temperature for 3 min. The progress of the reaction was followed by TLC. After the reaction completion, the products was extracted with EtOAc and filtered to remove Co3O4. The organic solvent was then washed with H2O (2 x 10 mL) and saturated NaHCO3 solution and then dried over anhydrous Na2SO4. The solvent was removed under vacuum to afford the pure product.
References:
Marjani, Ahmad Poursattar;Hosseini, Seyed Ali;Shokri, Zahra;Maleki, Nasim [Research on Chemical Intermediates,2017,vol. 43,# 1,p. 413 - 422]

122-85-0
231 suppliers
$10.00/1g

16375-88-5
111 suppliers
$22.00/1g

623-04-1
366 suppliers
$6.00/1g
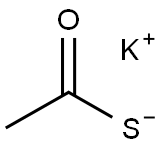
10387-40-3
367 suppliers
$10.00/1g

16375-88-5
111 suppliers
$22.00/1g