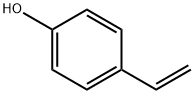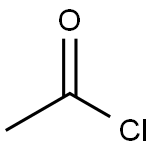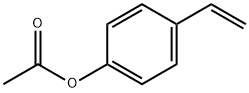
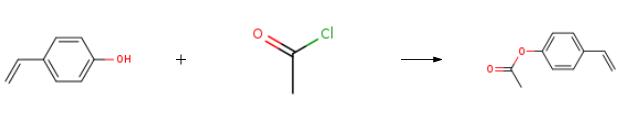
4-Acetoxystyrene synthesis
- Product Name:4-Acetoxystyrene
- CAS Number:2628-16-2
- Molecular formula:C10H10O2
- Molecular Weight:162.19
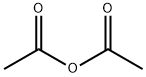
108-24-7
0 suppliers
$14.00/250ML

2628-16-2
401 suppliers
$7.00/1g
Yield:2628-16-2 97.5%
Reaction Conditions:
Stage #1: p-Coumaric Acidwith bis(1-oxyl-2,2,6,6-tetramethylpiperidin-4-yl)sebacate;potassium acetate in DMF (N,N-dimethyl-formamide) at 150; for 2 h;
Stage #2: acetic anhydride in DMF (N,N-dimethyl-formamide) at 140; for 0.75 h;Product distribution / selectivity;
Steps:
16; 17
The purpose of this Example was to prepare 4-acetoxystyrene using the thermal, base-catalyzed decarboxylation of para-hydroxycinnamic acid, followed by acetylation of the resulting 4-hydroxystyrene with acetic anhydride in a two-step, single vessel reaction. Prostab 5415 at a concentration of 100 ppm was used as the polymerization inhibitor. To a 3-neck round-bottom flask was added pHCA (5 g, 30.458 mmol, 1 eq), 30 mL of DMF (to make a 1 M solution in pHCA), 100 ppm Prostab 5415 (0.5 mg, added as 250 μL of a 2 mg/mL solution in DMF) and potassium acetate (30 mg, 1 mol %). The reaction was performed under nitrogen with stirring and with a reflux condenser. The reaction was heated to 150° C. with an oil bath and temperature controller (with overtemp protection). The potassium acetate was not soluble at room temperature, but dissolved as heat was applied, giving a pale yellow solution. The reaction was monitored by TLC, as described supra. After 2 h at 150° C., the reaction appeared to be complete, as determined by TLC. Then, the reaction temperature was reduced to 140° C. and acetic anhydride (4.32 mL, 45.687 mmol, 1.5 eq) was added dropwise to the reaction mixture, which was maintained at 140° C. The color of the reaction mixture became lighter. The reaction was complete after 0.75 h, as determined using TLC. Heating was discontinued and the reaction mixture was allowed to cool to room temperature. The reaction mixture was stored at -20° C. overnight. The 4-acetoxystyrene was isolated using the procedure described in Example 14 for the isolation of 4-hydroxystyrene, resulting in 5.39 g of a pale yellow, semi-solid (109% of the theoretical yield). The product was analyzed using 1H NMR. The greater than theoretical yield obtained and the nature of the product (i.e., pale yellow semi-solid) suggest that the product contained some impurities. 1H NMR (500 MHz, CDCl3): δ (ppm) 7.40 (2H, ABq, J=8.6 Hz), 7.045 (2H, ABq, J=8.5 Hz), 6.693 (1H, dd, J=17.5 and 11.0 Hz), 5.693 (1H, dd, J=17.6 and 0.7 Hz), 5.234 (1H, dd, J=10.9 and 0.8 Hz), 2.29 (2.64H, s) Example 17 Preparation of 4-Acetoxystyrene by Decarboxylation of Para-hydroxycinnamic Acid Followed by Acetylation in a Single Vessel Reaction The purpose of this Example was to prepare 4-acetoxystyrene using the thermal, base-catalyzed decarboxylation of para-hydroxycinnamic acid, followed by acetylation of the resulting 4-hydroxystyrene with acetic anhydride in a two-step, single vessel reaction. Prostab 5415 at a concentration of 1000 ppm was used as the polymerization inhibitor. The decarboxylation and acetylation reactions were carried out as described in Example 16, except that 1000 ppm Prostab 5415 (5 mg) was used as the polymerization inhibitor. The 4-acetoxystyrene product was isolated as described in Example 16, resulting in 4.82 g of a yellow oil (97.5% of the theoretical yield). The product was analyzed using 1H NMR. 1H NMR (500 MHz, CDCl3): δ (ppm) 7.40 (2H, ABq, J=8.4 Hz), 7.04 (2H, ABq, J=8.5 Hz), 6.695 (1H, dd, J=17.6 and 11.0 Hz), 5.695 (1H, d, J=17.6 Hz), 5.235 (1H, d, J=10.9 Hz), 2.29 (3H, s)
References:
US2005/228191,2005,A1 Location in patent:Page/Page column 8-9
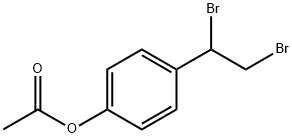
360068-17-3
0 suppliers
inquiry

2628-16-2
401 suppliers
$7.00/1g
![[4-(1-hydroxyethyl)phenyl] acetate](/CAS/GIF/53744-50-6.gif)
53744-50-6
10 suppliers
inquiry

2628-16-2
401 suppliers
$7.00/1g