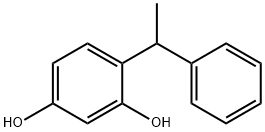
4-(alpha-Methylbenzyl)resorcinol synthesis
- Product Name:4-(alpha-Methylbenzyl)resorcinol
- CAS Number:85-27-8
- Molecular formula:C14H14O2
- Molecular Weight:214.26
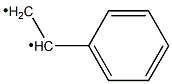
292638-84-7
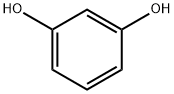
108-46-3

85-27-8
1) Under nitrogen protection, 231 g (2.1 mol) of resorcinol, 4.62 g of the solid acid H2SO4-SiO2, and 220 mL of toluene were added to a reactor, heated to 95 °C, and maintained at that temperature. Subsequently, 104 g (1 mol) of styrene was slowly added dropwise to the reactor at a controlled rate for 1 hour. After completion of the dropwise addition, the reaction was continued at 95 °C for 1 h to obtain the reaction mixture. 2) After completion of the reaction, the reaction mixture was filtered to remove the solid acid H2SO4-SiO2. 150 mL of 0.5% aqueous sodium bicarbonate was added to the filtrate for extraction to separate the organic phase. The organic phase was washed twice more (150 mL each) with deionized water. The organic phase was collected and toluene was removed by rotary evaporator. Subsequently, 250 mL of a mixed solution of hexane and toluene (1:5, v/v) was added for recrystallization, and 177.6 g of white crystal product was obtained in 83% yield.
Yield:85-27-8 88%
Reaction Conditions:
in toluene at 95;Sealed tube;Inert atmosphere;Large scale;Concentration;Temperature;
Steps:
1; 1.1; 1.2; 2; 3; 3.1; 3.2; 4 Example 3
(1) Add 50 L of toluene to the reaction vessel, start stirring, and add 39.6 kg (360 mol) of resorcinol (mw: 110.11).0.45 kg of catalyst solid acid H2SO4-SiO2 (by mass ratio H2SO4: SiO2 = 1:1),After sealing, the vacuum was evacuated and replaced with nitrogen three times. Under a nitrogen atmosphere, the reactor was heated to 95 ° C.After the temperature was stable, 15 kg (144 mol) of styrene (mw: 104.15) was slowly dropped.Control the feeding time 1.5-2 hours, keep the reaction for 3 hours,The reaction was completely detected by liquid phase, and the yield was 89%, and the reaction was stopped.
(2) adding 10 liters of toluene to the reaction solution,Recovery of solid acid H2SO4-SiO2 catalyst by hot filtration,The filtrate was slowly cooled to 0 ° C under stirring, and solid resorcinol was precipitated, filtered, and solid resorcinol was washed with 12 liters (6 liters of X 2 times) of toluene for recovery.The filtrate was concentrated until a solid precipitated, and 10 liters of a mixed solvent (by volume ratio, n-hexane: toluene = 1:1) was heated to 75 ° C to dissolve sufficiently.After cooling to -5 ° C under stirring, crystals were precipitated, and the mixture was kept for 2 hours until the crystals were completely precipitated, followed by pressure filtration, and the obtained solid was washed twice with 20 liters of a mixed solvent (by volume ratio, n-hexane: toluene = 1:1).The resulting solid was dried under vacuum at 50 ° C.It has a white crystal of 24.2 kg and a liquid phase detection content of 98.4%.The product yield is 88%, and the product has a melting point of 76-78 °C.
References:
Liaoning University;Zhou Yunpeng;Zhang Qiushi CN109534960, 2019, A Location in patent:Paragraph 0018; 0025-0041