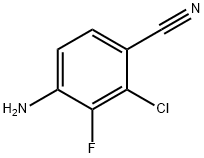
4-Amino-2-chloro-3-fluorobenzonitrile synthesis
- Product Name:4-Amino-2-chloro-3-fluorobenzonitrile
- CAS Number:757247-99-7
- Molecular formula:C7H4ClFN2
- Molecular Weight:170.57
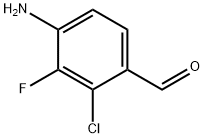
757247-98-6
22 suppliers
$155.00/50mg

757247-99-7
100 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 4-amino-2-chloro-3-fluoro-benzaldehydewith hydroxylamine hydrochloride in pyridine;water at 20; for 1 h;
Stage #2: with copper(II) sulfate;triethylamine in pyridine;dichloromethane;water;
Stage #3: with formic acid;dicyclohexyl-carbodiimidemore than 3 stages;
Steps:
9.9B
To a solution of hydroxylamine hydrochloride (336 mg, 4.84 mmol) in water (1.2 mL) was added 4-amino-2-chloro-3-fluoro-benzaldehyde (9A) (0.800 g, 4.61 mmol) and pyridine (2.5 mL). After stirring at rt for 1 h, copper (H) sulfate pentahydrate (230 mg, 0.922 mmol) was added followed by a solution of triethylamine (1.40 mL, 9.68 mmol) in CH2Cl2 (2.5 mL). To the resulting dark green reaction mixture was then added a solution of DCC (1.14 g, 5.53 mmol) in CH2Cl2 (10 mL) and the reaction was stirred for 2 h. Formic acid (1 mL) was added and the reaction was stirred for 20 min, filtered through celite, and concentrated. The resulting residue was purified via silica gel chromatography eluding with 20-30% EtOAc/hexane to provide the title compound (0.780 g) as a light brown solid.
References:
US2005/197359,2005,A1 Location in patent:Page/Page column 29
2106-04-9
257 suppliers
$6.00/10g

757247-99-7
100 suppliers
inquiry
143360-05-8
6 suppliers
inquiry

757247-99-7
100 suppliers
inquiry
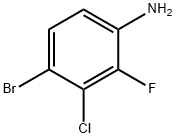
115843-99-7
115 suppliers
$10.00/1g

757247-99-7
100 suppliers
inquiry
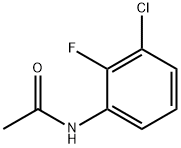
395-36-8
7 suppliers
inquiry

757247-99-7
100 suppliers
inquiry