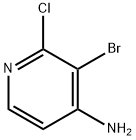
4-AMINO-3-BROMO-2-CHLOROPYRIDINE synthesis
- Product Name:4-AMINO-3-BROMO-2-CHLOROPYRIDINE
- CAS Number:215364-85-5
- Molecular formula:C5H4BrClN2
- Molecular Weight:207.46
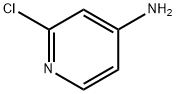
14432-12-3

215364-85-5
General procedure for the synthesis of 3-bromo-2-chloropyridin-4-amine from 2-chloro-4-aminopyridine: N-bromosuccinimide (NBS, 2.77 g, 15.56 mmol) was added batchwise to a stirred solution of 2-chloropyridin-4-amine (17A, 2 g, 15.56 mmol) in acetic acid (20 mL) at 0 °C and under nitrogen protection. The reaction mixture was then stirred at room temperature for 1 hour. Upon completion of the reaction, the solvent was removed by distillation under reduced pressure and azeotropic distillation with ethanol was performed to further remove the residual solvent. The crude product was purified by silica gel column chromatography (using a 40 g RediSep column with a gradient elution using a petroleum ether solution of 10-20% ethyl acetate as eluent) to afford the target compound, 3-bromo-2-chloropyridin-4-amine (15B, 2 g, 9.64 mmol, 62.0% yield), as a white solid.LCMS analysis: m/z = 209.0 [M + H]+; retention time 0.84 min; condition B.

14432-12-3
541 suppliers
$5.00/5g

215364-85-5
140 suppliers
$11.00/250mg
Yield:215364-85-5 62%
Reaction Conditions:
with N-Bromosuccinimide;acetic acid at 0 - 20; for 1 h;Inert atmosphere;
Steps:
Compound 17B: 3-bromo-2-chloropyridin-4-amine
To a stirred solution of 2-chloropyridin-4-amine 17A (2 g, 15.56 mmol) in acetic acid (20 mL) was added NBS (2.77 g, 15.56 mmol) portion-wise at 00 C under nitrogen. Then reaction mixture was allowed to stir at room temperature for 1 h. The solvent was removed under reduced pressure, followed by azeotropic distillation with ethanol. The cmde compound was purified by silica gel chromatography (40 g RediSep column, eluting with a gradient of 10-20 % ethyl acetate in petroleum ether) to yield Compound 15B (2 g, 9.64 mmol, 62.0 % yield) as a white solid. LCMS: m/z = 209.0 [M+Hf’; ret. time 0.84 mm; condition B.
References:
RIGEL PHARMACEUTICALS, INC.;BRISTOL-MYERS SQUIBB COMPANY;DING, Pingyu;GELMAN, Marina;KINSELLA, Todd;SINGH, Rajinder;BHAMIDIPATI, Somasekhar;VELAPARTHI, Upender;BORZILLERI, Robert, M.;RAHAMAN, Hasibur;WARRIER, Jayakumar, Sankara WO2016/133838, 2016, A1 Location in patent:Paragraph 0315

14432-12-3
541 suppliers
$5.00/5g
857730-21-3
181 suppliers
$45.00/50mg

215364-85-5
140 suppliers
$11.00/250mg