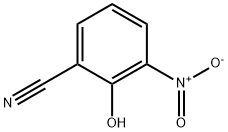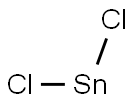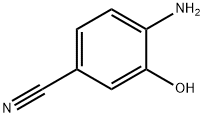
4-AMINO-3-HYDROXY-BENZONITRILE synthesis
- Product Name:4-AMINO-3-HYDROXY-BENZONITRILE
- CAS Number:55586-26-0
- Molecular formula:C7H6N2O
- Molecular Weight:134.14
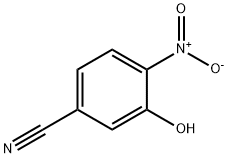
18495-15-3

55586-26-0
The general procedure for the synthesis of 4-amino-3-hydroxybenzonitrile from 3-hydroxy-4-nitrobenzonitrile was as follows: 3-hydroxy-4-nitrobenzonitrile (1.6 g, 9.6 mmol) was dissolved in a solvent mixture of ethanol (40 mL) and N,N-dimethylformamide (20 mL). Palladium hydroxide (30 mg) was added to this solution to form a reaction mixture. The reaction mixture was stirred overnight at room temperature in a hydrogen atmosphere. Upon completion of the reaction, the reaction mixture was filtered to remove the catalyst and the solvent was subsequently removed by distillation under reduced pressure. The residue was dried to afford crystals of 4-amino-3-hydroxybenzonitrile (771 mg, 60% yield). The product was characterized by 1H-NMR (DMSO-d6) with the following chemical shifts: δ 5.57 (2H, broad peak), 6.62 (1H, double peak, J = 8.4 Hz), 6.86 (1H, double peak, J = 2.2 Hz), and 6.99 (1H, double-double peak, J = 1.9 Hz, 8.1 Hz).
Yield: 77%
Reaction Conditions:
in acetic acid
Steps:
5 b)Preparation of 2-amino-5-cyanophenol
b)Preparation of 2-amino-5-cyanophenol A mixture of 6cyano2-nitrophenol(2.40 g, 14.6 mmol) and tin (II) chloride (9.6 g, 43.2 mmol) in acetic acid(100 mL) was heated at 80° C. under argon. After 2 hours, the starting material had disappeared and the solution was allowed to cool down and then poured into ice. The pH was made slightly basic (pH 7-8), by addition of solid NaOH, before being extracted with ethyl acetate. The organic phase was washed with brine, dried over MgSO4 and filtered. The solvent was evaporated and chromatography of the resulting solid on silica gel (4%MeOH/CH2Cl2) gave the desired product(1.50 g, 77%). 1H NMR (CD3OD): δ6.92 (d, 1H), 6.85-6.69 (m,2H).
References:
SmithKline Beecham Corporation US6271261, 2001, B1

18495-15-3
63 suppliers
inquiry

55586-26-0
69 suppliers
inquiry
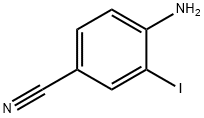
33348-34-4
136 suppliers
$10.00/1g

55586-26-0
69 suppliers
inquiry
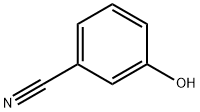
873-62-1
261 suppliers
$6.00/1g

55586-26-0
69 suppliers
inquiry