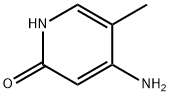
4-Amino-2-hydroxy-5-methylpyridine synthesis
- Product Name:4-Amino-2-hydroxy-5-methylpyridine
- CAS Number:95306-64-2
- Molecular formula:C6H8N2O
- Molecular Weight:124.14

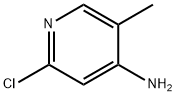
79055-62-2
215 suppliers
$10.00/250mg

95306-64-2
283 suppliers
$31.00/250mg
Yield:95306-64-2 84%
Reaction Conditions:
with potassium hydroxide in methanol at 180; under 9375.94 Torr; for 16 h;
Steps:
2 Example 2 Preparation of 4-amino-5-methyl-1H-pyridin-2-One (I)
A pressure reactor was charged with 4.0 g of the title compound from Example 1 (compound 2) in 40 ml of methanol and 12.5 g of potassium hydroxide (KOH) was added. This was then heated to 180° C. for 16 hours (rise in pressure to 12.5 bar). It was allowed to cool. (0055) The reaction was carried out 5 times with in each case 4.0 g of the title compound from Example 1 and the reaction solutions combined after cooling. (0056) Workup: The mixture was adjusted to pH 7.0 with approx. 100 ml of aq. 25% hydrochloric acid while cooling, then evaporated to dryness under reduced pressure, and the residue azeotroped 5 times with ethanol, each time with 50 ml (evaporated to dryness under reduced pressure to remove traces of water). 400 ml of methanol was added to the evaporation residue and the mixture was stirred. The salt (KCl) was filtered off and washed with two 25 ml portions of methanol. The filtrate was concentrated to dryness under reduced pressure. The evaporation residue was recrystallized from 60 ml of water. After cooling to 0° C., the precipitated crystals were filtered off. The wet product was then dried under reduced pressure at 30° C. (0057) Yield: 13.5 g (77.53% of theory); purity according to HPLC: 99.1% (0058) A further 1.10 g (6.32% of theory) was isolated from the mother liquor, thereby achieving an overall yield of approx. 84% of theory. (0059) MS (EIpos): m/z=125 [M+H]+ (0060) 1H-NMR (300 MHz, DMSO-d6): δ=1.81 (s, 3H), 2.54 (s, 1H), 5.24 (s, 1H), 5.79 (s, 2H), 6.85 (s, 1H), 10.27 (br s, 1H)
References:
US2022/153699,2022,A1 Location in patent:Paragraph 0054-0060
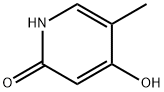
41935-71-1
25 suppliers
inquiry

95306-64-2
283 suppliers
$31.00/250mg
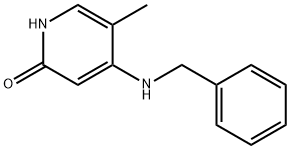
95306-62-0
1 suppliers
inquiry

95306-64-2
283 suppliers
$31.00/250mg

41935-71-1
25 suppliers
inquiry

71-36-3
1092 suppliers
$14.00/25mL

95306-64-2
283 suppliers
$31.00/250mg
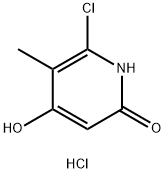
95306-65-3
5 suppliers
inquiry

95306-64-2
283 suppliers
$31.00/250mg