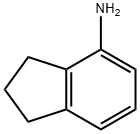
4-AMINOINDAN synthesis
- Product Name:4-AMINOINDAN
- CAS Number:32202-61-2
- Molecular formula:C9H11N
- Molecular Weight:133.19
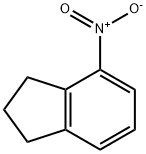
34701-14-9

32202-61-2
General procedure for the synthesis of 4-aminoindan from 4-nitro-2,3-dihydro-1H-indene: 4-nitroindan (10 g, 61 mmol) was dissolved in 50 mL of ethanol in a 500 mL Parr shaker vessel. A 10% Pd/C (1 g) ethanol slurry was added. The mixture was subjected to a hydrogen atmosphere (50 psi) and reacted on a Parr oscillator for 1 h. The progress of the reaction was monitored by thin-layer chromatography (TLC, unfolding agent was a hexane solution of 20% ethyl acetate) to confirm that the feedstock was completely consumed. Upon completion of the reaction, the mixture was filtered twice through a diatomaceous earth pad and washed with a large amount of ethanol and filtered once more through filter paper. The ethanol was removed by evaporation under reduced pressure and the resulting crude product was purified by fast column chromatography on silica gel (eluent was a hexane solution of 10% ethyl acetate) to afford the target compound 4-aminoindan (8) as a viscous colored oil (7.04 g, 86% yield). Its 1H NMR (400 MHz, DMSO-d6) data were as follows: δ 1.95 (m, 2H), 2.61 (t, J = 7.3 Hz, 2H), 2.76 (t, J = 7.5 Hz, 2H), 4.77 (s, 2H), 6.36 (d, J = 7.8 Hz, 1H), 6.42 (d, J = 6.8 Hz, 1H), 6.80 (t, J = 7.6 Hz, 1H).

34701-14-9
42 suppliers
$80.40/5g

32202-61-2
109 suppliers
$29.00/1g
Yield:32202-61-2 86%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in ethanol under 2585.81 Torr; for 1 h;
Steps:
5 Intermediate 8: 4-aminoindane
Intermediate 8: 4-aminoindane In a 500 mL Parr shaker vessel, 4-nitroindane (10 g, 61 mmol) was dissolved in 50 mL ethanol. A slurry of 10% Pd/C (1 g) in ethanol was added. The mixture was then placed on a Parr shaker under a hydrogen atmosphere (50 psi) for 1 hour, at which point t.l.c. (20% ethyl acetate in hexanes) showed that all the starting material had disappeared. To work up the reaction, the mixture was filtered twice through Celite, washing with a large amount of ethanol, and once through filter paper. The ethanol was evaporated under reduced pressure, and the crude product purified by flash chromatography over silica gel (10% ethyl acetate in hexanes) to give 8 as a viscous, faintly colored oil (7.04 g, 86% yield): 1H NMR (400 MHz, DMSO-D6) δ 1.95 (m, 2H) 2.61 (t, J=7.3 Hz, 2H) 2.76 (t, J=7.5 Hz, 2H) 4.77 (s, 2H) 6.36 (d, J=7.8 Hz, 1H) 6.42 (d, J=6.8 Hz, 1H) 6.80 (t, J=7.6 Hz, 1H).
References:
Wyeth US2005/101569, 2005, A1 Location in patent:Page/Page column 17
496-11-7
256 suppliers
$5.00/5g

32202-61-2
109 suppliers
$29.00/1g
4044-54-6
44 suppliers
$177.00/100mg

32202-61-2
109 suppliers
$29.00/1g
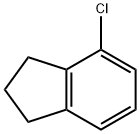
15115-61-4
15 suppliers
inquiry

32202-61-2
109 suppliers
$29.00/1g
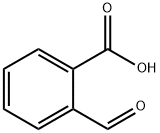
119-67-5
379 suppliers
$2.00/1g

32202-61-2
109 suppliers
$29.00/1g