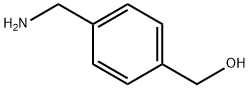
(4-AMINOMETHYL-PHENYL)-METHANOL synthesis
- Product Name:(4-AMINOMETHYL-PHENYL)-METHANOL
- CAS Number:39895-56-2
- Molecular formula:C8H11NO
- Molecular Weight:137.18
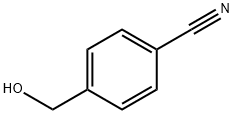
874-89-5

39895-56-2
A reduction reaction was carried out using 4-hydroxymethylbenzonitrile (2.0 g, 15 mmol) as a raw material in MeOH:NH3 (100 ml) solution under hydrogen (50 psi) atmosphere for 20 hrs using nickel Nguyenay (0.5 g) as a catalyst. Upon completion of the reaction, the reaction mixture was filtered through a diatomaceous earth pad to remove the catalyst. The filtrate was concentrated under reduced pressure to afford a white solid (4-aminomethylphenyl) methanol hydrochloride (2.01 g, 98% yield), which was pure enough to be used directly in the subsequent reaction without further purification. The product characterization data were as follows: mass spectrum (APCI+): m/z 138.3 (M+H)+; 1H-NMR (DMSO-d6) δ 7.20 (s, 4H), 5.04 (s, 2H), 4.42 (s, 2H), 3.83 (s, 1H), 2.45 (s, 2H).
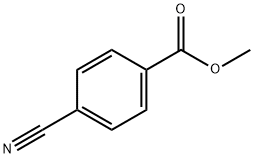
1129-35-7
303 suppliers
$8.00/5g

39895-56-2
145 suppliers
$15.00/100mg
Yield: 80%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0 - 50; for 15 h;
Steps:
336.1 Step 1: (4-(Aminomethyl)phenyl)methanol
To a suspension of lithium aluminium hydride in tetrahydrofuran (400 mL) stirred in air at 0 °C was added a solution of methyl 4-cyanobenzoate (10 g, 62.1 mmol) in tetrahydrofuran (400 mL) dropwise over 15 minutes. The reaction mixture was stirred at 50 °C for 15 hours and then cooled to 0°C and quenched by the slow addition of water (7 mL), 15% NaOH (7 mL), and water (21 mL). The resulting precipitate was stirred for an additional 30 minutes and filtered. The filtrated was concentrated in vacuo to give (4- (aminomethyl)phenyl)methanol (6.8 g, 80% yield) as a light yellow oil. LCMS m/z = 138.0 [M+H]+.
References:
GLAXOSMITHKLINE INTELLECTUAL PROPERTY DEVELOPMENT LIMITED;ADAMS, Nicholas David;BENOWITZ, Andrew B.;RUEDA BENEDE, Maria Lourdes;EVANS, Karen Anderson;FOSBENNER, David T.;KING, Bryan Wayne;LI, Mei;MILLER, William Henry;REIF, Alexander Joseph;ROMERIL, Stuart Paul;SCHMIDT, Stanley J.;WIGGALL, Kenneth WO2017/216726, 2017, A1 Location in patent:Page/Page column 941

874-89-5
176 suppliers
$6.00/250mg

39895-56-2
145 suppliers
$15.00/100mg
56-91-7
542 suppliers
$5.00/1g

39895-56-2
145 suppliers
$15.00/100mg
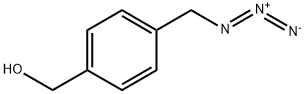
439691-96-0
1 suppliers
inquiry

39895-56-2
145 suppliers
$15.00/100mg
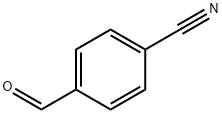
105-07-7
394 suppliers
$5.00/5g

39895-56-2
145 suppliers
$15.00/100mg