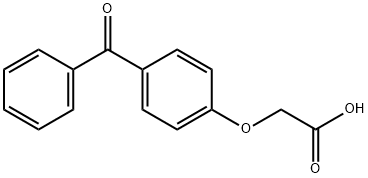
(4-BENZOYL-PHENOXY)-ACETIC ACID synthesis
- Product Name:(4-BENZOYL-PHENOXY)-ACETIC ACID
- CAS Number:6322-83-4
- Molecular formula:C15H12O4
- Molecular Weight:256.25
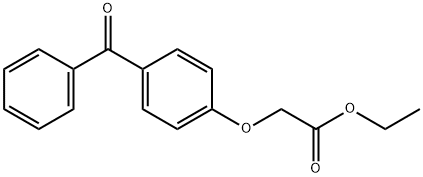
51848-56-7

6322-83-4
General procedure for the synthesis of (4-benzoylphenoxy)-acetic acid from ethyl 2-(4-benzoylphenoxy)acetate: 1N NaOH solution (3.9 mmol) was added to an ethanol (20 mL) solution of ethyl 2-(4-benzoylphenoxy)acetate (3.0 mmol) and the reaction mixture was stirred for 10-15 h at room temperature. Upon completion of the reaction, the solvent was removed by distillation under reduced pressure and the residue was dissolved in water (20 mL) and acidified with concentrated HCl at 0 °C. Subsequently, the aqueous layer was extracted with dichloromethane (3 x 20 mL), the organic layers were combined and dried with anhydrous Na2SO4. After drying, the organic layer was concentrated under reduced pressure to give the crude product. Finally, the crude product was purified by crystallization from cyclohexane or chloroform to afford the target compound (4-benzoylphenoxy)-acetic acid in good yield.

51848-56-7
15 suppliers
inquiry

6322-83-4
46 suppliers
$12.00/100mg
Yield: 74%
Reaction Conditions:
Stage #1:(4-benzoylphenoxy)acetic acid ethyl ester with sodium hydroxide in ethanol at 20;
Stage #2: with hydrogenchloride in water at 0;
Steps:
General procedure for the preparation of acids 17-26.
General procedure: A solution of NaOH 1N (3.9 mmol) was added to esters 7-16(3.0 mmol) in EtOH (20 mL), and the mixture was stirred at r.t. for 10-15 h. The solvent was removed under reducedpressure and the residue was poured into water (20 mL) andacidified with conc HCl at 0 °C. The aqueous layer was extractedwith dichloromethane (3 20 mL) and then the organiclayer was dried over Na2SO4 and concentrated underreduced pressure. The residue was purified by crystallizationwith cyclohexane or chloroform affording desired acids 17-26 with good yields.
References:
Giampietro, Letizia;D'Angelo, Alessandra;Giancristofaro, Antonella;Ammazzalorso, Alessandra;De Filippis, Barbara;Di Matteo, Mauro;Fantacuzzi, Marialuigia;Linciano, Pasquale;Maccallini, Cristina;Amoroso, Rosa [Medicinal Chemistry,2014,vol. 10,# 1,p. 59 - 65]
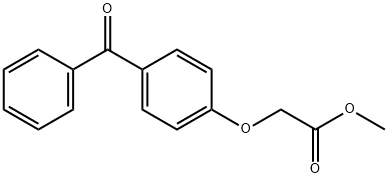
57682-09-4
11 suppliers
inquiry

6322-83-4
46 suppliers
$12.00/100mg
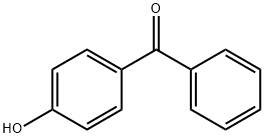
1137-42-4
512 suppliers
$6.00/25g

6322-83-4
46 suppliers
$12.00/100mg
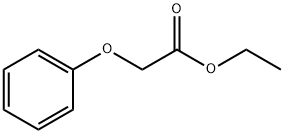
2555-49-9
148 suppliers
$19.00/5 g

6322-83-4
46 suppliers
$12.00/100mg
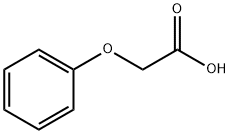
122-59-8
456 suppliers
$5.00/25g

6322-83-4
46 suppliers
$12.00/100mg