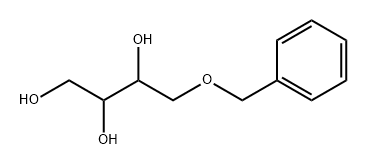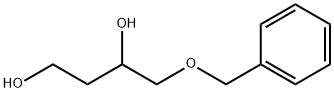
4-Benzyloxy-1,3-butanediol synthesis
- Product Name:4-Benzyloxy-1,3-butanediol
- CAS Number:71998-70-4
- Molecular formula:C11H16O3
- Molecular Weight:196.24
Yield:71998-70-4 69%
Reaction Conditions:
with copper(l) iodide;methyllithium in diethyl ether at -40 - 0; for 3 h;Inert atmosphere;regioselective reaction;
Steps:
To a stirred suspension of CuI (14.7 g, 74.2 mmol) in anhydrous Et2O (147 mL) at 0 °C was added slowly MeLi (92.7 mL, 0.148 mmol, 1.6 M in Et2O) under an N2 atm, and the resulting solution was stirred for 15 min at 0 °C. Epoxy alcohol 16 (4.8 g, 24.7 mmol) in anhydrous Et2O (20 mL) was then added dropwise at -40 °C. Once the addition was complete, the mixture was stirred at 0 °C for 3 h. The reaction was quenched by the careful addition of sat. aq NH4Cl solution. The mixture was filtered through a pad of Celite, and the salts were washed several times with Et2O. The combined organic layers were washed with brine (2 × 20 mL) and dried over Na2SO4. Concentration of the Et2O extract under reduced pressure provided 4.9 g (23.4 mmol, 94%) of a pale yellow oil, which was a 6:1 mixture of the regioisomeric diols. This mixture was dissolved in THF (60 mL) and treated with H2O (30 mL) containing NaIO4 (7.4 g, 34.7 mmol) with stirring to cleave the 1,2-diol. The reaction was complete in 1 h. After the layers were separated, the aq layer was extracted with Et2O (3 × 30 mL) and the combined extracts dried over Na2SO4 and concentrated in vacuo. The residue was subjected to silica gel column chromatography (EtOAc-hexane, 4:6) to afford diol 17 (3.5 g, 69%) as a pale yellow liquid.
References:
Sabitha, Gowravaram;Gurumurthy;Yadav, Jhillu Singh [Synthesis,2014,vol. 46,# 1,art. no. SS-2013-Z0529-OP,p. 110 - 118]
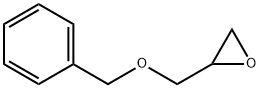
2930-05-4
127 suppliers
$8.00/5g

71998-70-4
28 suppliers
$60.00/100mg
![2-Oxiranemethanol, 3-[(phenylmethoxy)methyl]-, (2R,3R)-](/CAS/20210305/GIF/78513-15-2.gif)