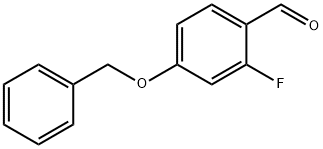
4-(Benzyloxy)-2-fluorobenzaldehyde synthesis
- Product Name:4-(Benzyloxy)-2-fluorobenzaldehyde
- CAS Number:504414-32-8
- Molecular formula:C14H11FO2
- Molecular Weight:230.23
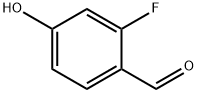
348-27-6

100-39-0

504414-32-8
Example 2: Synthesis of 10-acetyl-1-(4-benzyloxy-2-fluorophenyl)-3,3-dimethyl-1,1-dioxo-1,2,3,4,5,11-hexahydro-1,4,6-thia-5,10-diazadibenzo[α,π]cycloheptene (13); Step 1: In anhydrous N,N-dimethylformamide (DMF, 10 mL), a 2-fluoro-4 -hydroxybenzaldehyde (2.92 mmol), benzyl bromide (534 μL, 4.38 mmol) and cesium carbonate (Cs2CO3, 1.14 g, 3.50 mmol) in an anhydrous N,N-dimethylformamide (DMF, 10 mL) was stirred at room temperature for 3 days. Upon completion of the reaction, the reaction mixture was diluted with water (200 mL), the resulting precipitate was collected by filtration and dried under vacuum to afford the target product 4-benzyloxy-2-fluorobenzaldehyde (11) in 94.4% yield as a white solid: m/z = 231(M + H)+.

348-27-6
207 suppliers
$15.00/1g

100-39-0
432 suppliers
$10.00/10g

504414-32-8
57 suppliers
$42.00/250mg
Yield:504414-32-8 94.4%
Reaction Conditions:
with caesium carbonate in N,N-dimethyl-formamide at 20; for 72 h;
Steps:
2.1
Example 2: 10-acetyl-l l-(4-benzyloxy-2-fluorophenyl)-3,3-dimethyl-l,l-dioxo- 1,2,3,4,5,1 l-hexahvdro-lλ6-thia-5J0-diazadibenzorα,?cvcloheptene (13); Step 1; A mixture of 2-fluoro-4-hydroxybenzaldehyde (2.92 mmol), benzylbromide (534 μL, 4.38 mmol) and Cs2CO3 (1.14 g, 3.50 mmol) in dry DMF (N,N-dimethylformamide;
References:
WO2008/99020,2008,A1 Location in patent:Page/Page column 49-50

185836-35-5
37 suppliers
inquiry

504414-32-8
57 suppliers
$42.00/250mg

504414-33-9
5 suppliers
inquiry

504414-32-8
57 suppliers
$42.00/250mg