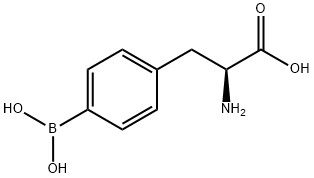
4-BORONO-L-PHENYLALANINE synthesis
- Product Name:4-BORONO-L-PHENYLALANINE
- CAS Number:76410-58-7
- Molecular formula:C9H12BNO4
- Molecular Weight:209.01
119771-23-2

76410-58-7
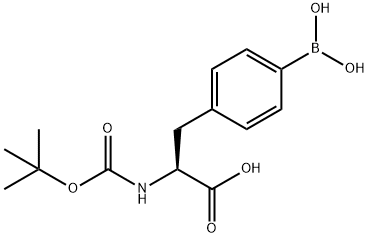
The general procedure for the synthesis of (S)-2-amino-3-(4-boronic acid phenyl)propionic acid from (S)-3-(4-boronic acid phenyl)-2-((tert-butoxycarbonyl)amino)propionic acid was as follows: (S)-N-Boc-4-borophenylalanine (5.63 g, 98.5% purity, 17.9 mmol) was suspended in a solvent mixture of acetone (34 mL) and water (3.8 mL). Concentrated hydrochloric acid (37%, 3.8 mL) was added slowly under stirring to form an acidic reaction system. The reaction system was stirred at 55 °C for 1.5 h. HPLC showed that the reaction was complete. The reaction system was cooled to room temperature and the pH was adjusted to 1.5 with aqueous sodium hydroxide solution and stirred for 30 min, during which (S)-2-amino-3-(4-boronic acid phenyl)propionic acid started to precipitate. Subsequently, the pH of the reaction system was re-adjusted to 6.2 with aqueous sodium hydroxide and stirring was continued overnight at room temperature. Upon completion of the reaction, the solid product was collected by filtration to afford (S)-2-amino-3-(4-boronic acid phenyl)propionic acid.

119771-23-2
82 suppliers
$70.00/5g

76410-58-7
190 suppliers
$21.00/100mg
Yield:76410-58-7 93.2%
Reaction Conditions:
with hydrogenchloride in water;acetone at 55; for 1.5 h;
Steps:
1 Preparation of 4-borono-L-phenylalanine (L-BPA) from (S)-N-Boc-4-boronophenylalanine
A suspension of (S)-N-Boc-4-boronophenylalanine(5.63 g, 98.5% pure, 17.9 mmol) in a mixture of acetone (34 ml) and water (3.8 ml) was stirred and added hydrochloric acid (37 %, 3.8 ml) to form an acidic mixture, and the acidic mixture was stirred at 55°C for 1.5 h. HPLC analysis of the acidic mixture showed the completion of the reaction.
The acidic mixture was cooled to room temperature, and the pH value of the acidic mixture was adjusted to pH 1.5 by using sodium hydroxide aqueous solution.
The acidic mixture was stirred for 30 min, and the product 4-borono-L-phenylalanine started to precipitate during this period.
The pH value of the acidic mixture was readjusted to pH 6.2 by using sodium hydroxide aqueous solution, and the acidic mixture was stirred overnight at room temperature.
The acidic mixture was filtered to obtain solid 4-borono-L-phenylalanine.
References:
Taiwan Biotech Co., Ltd.;Sheu, Kuen-Wang;Huang, Shu-Fen;Shaw, Chia-Cheng EP2865682, 2015, A1 Location in patent:Paragraph 0077; 0078

262604-05-7
0 suppliers
inquiry

76410-58-7
190 suppliers
$21.00/100mg
![L-Phenylalanine, 4-borono-N-[(phenylmethoxy)carbonyl]- (9CI)](/CAS/20200611/GIF/164203-33-2.gif)
164203-33-2
0 suppliers
inquiry

76410-58-7
190 suppliers
$21.00/100mg

220400-04-4
50 suppliers
$80.00/250mg

76410-58-7
190 suppliers
$21.00/100mg

1333-74-0
116 suppliers
$190.00/56L
![L-Phenylalanine, 4-borono-N-[(phenylmethoxy)carbonyl]- (9CI)](/CAS/20200611/GIF/164203-33-2.gif)
164203-33-2
0 suppliers
inquiry

76410-58-7
190 suppliers
$21.00/100mg