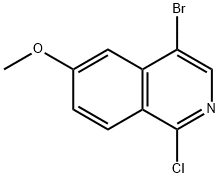
4-bromo-1-chloro-6-methoxyIsoquinoline synthesis
- Product Name:4-bromo-1-chloro-6-methoxyIsoquinoline
- CAS Number:1409964-75-5
- Molecular formula:C10H7BrClNO
- Molecular Weight:272.53
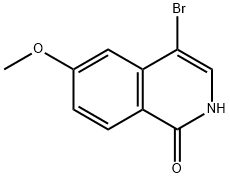
923278-23-3
12 suppliers
inquiry

1409964-75-5
15 suppliers
inquiry
Yield:1409964-75-5 65%
Reaction Conditions:
with trichlorophosphateReflux;
Steps:
4 Preparation of 4-bromo-1-chloro-6-methoxyisoquinoline
A solution of 4-bromo-6-methoxyisoquinolin-1(2H)-one (1.5 g, 5.90 mmol) in POCl3(15 ml) was refluxed for overnight. The solvent was evaporated under reduced pressure and the residue was diluted with cold water. The aqueous solution was basified by solid sodium carbonate and extracted with ethyl acetate. The combine organic layer was dried over anhydrous sodium sulfate, filtered and evaporated under reduced pressure to get crude compound. The crude compound was purified by silica gel chromatography (10% ethyl acetate in pet ether) to get desired compound (1.1 g, 65%) as white solid.1H NMR (400 MHz, DMSO-d6): δ ppm 8.53 (s, 1H), 8.27-8.24 (d, J=12 Hz, 1H), 7.56-7.53 (d, J=12 Hz, 1H), 7.41 (s, 1H), 4.02 (s, 3H); MS: MS m/z 273.99 (M++1).
References:
US9527885,2016,B2 Location in patent:Page/Page column 575-576

872496-89-4
0 suppliers
inquiry

1409964-75-5
15 suppliers
inquiry
6099-04-3
146 suppliers
$10.00/5g

1409964-75-5
15 suppliers
inquiry
26829-43-6
59 suppliers
$27.00/100mg

1409964-75-5
15 suppliers
inquiry

26829-43-6
59 suppliers
$27.00/100mg

1409964-75-5
15 suppliers
inquiry