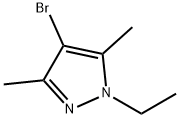
4-bromo-1-ethyl-3,5-dimethyl-1H-pyrazole synthesis
- Product Name:4-bromo-1-ethyl-3,5-dimethyl-1H-pyrazole
- CAS Number:51108-51-1
- Molecular formula:C7H11BrN2
- Molecular Weight:203.08
17629-26-4
65 suppliers
$185.00/1g

51108-51-1
16 suppliers
$58.00/100mg
Yield:51108-51-1 74%
Reaction Conditions:
with oxone;sodium bromide in water at 20; for 2 h;
Steps:
Representative Procedure for Pyrazole Halogenation
General procedure: 1-benzyl-4-chloro-3,5-dimethyl-1H-pyrazole. To a 16 mL vial containing 1-benzyl-3,5-dimethyl-1H-pyrazole (196 mg, 1.05 mmol) and a magnetic stir bar, 0.7 mL of water and 0.3 mL of ethyl acetate was added. Next, NaCl (123 mg, 2 mmol) was added and the vial was placed in a room temperature water bath to control exotherms. Finally, Oxone (322 mg, 0.52 mmol or 1.05 mmol KHSO5) was added and the vial was capped. The reaction proceeded with continuous and vigorous stirring until no starting material remained as indicated by TLC (1 h). The remaining oxidants were reduced with solid sodium bisulfite until starch iodide paper tested negative. Water (5 mL) was added and the mixture was extracted with 1:1 hexanes/diethyl ether (3 x 5 mL). The combined organic fractions were dried (MgSO4) and concentrated to yield crude product that was purified by flash chromatography (14 x 1 cm), 9:1 hexane/ethyl acetate eluent. Pure 1-benzyl-4-chloro-3,5-dimethyl-1H-pyrazole was obtained as a pale yellow oil (215 mg, 93% yield).
References:
Olsen, Kathryn L.;Jensen, Matthew R.;MacKay, James A. [Tetrahedron Letters,2017,vol. 58,# 43,p. 4111 - 4114] Location in patent:supporting information