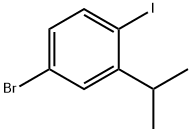
4-BroMo-1-iodo-2-isopropylbenzene synthesis
- Product Name:4-BroMo-1-iodo-2-isopropylbenzene
- CAS Number:1147014-97-8
- Molecular formula:C9H10BrI
- Molecular Weight:324.98
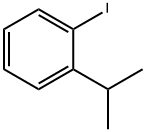
19099-54-8

1147014-97-8
General procedure for the synthesis of 5-bromo-2-iodoisopropylbenzene from 2-isopropyliodobenzene: 1-iodo-2-isopropylbenzene (1.5 g, 6.1 mmol) was mixed with silver sulfate (1.0 g, 3.1 mmol), concentrated sulfuric acid (5.5 mL), water (0.6 mL) and bromine (0.3 mL, 6.4 mmol) to form a three-phase suspension. The reaction mixture was stirred at room temperature for 1 hour. Upon completion of the reaction, the mixture was poured into a mixture of ether (150 mL) and water (75 mL). The solids were removed by filtration and the organic and aqueous layers were separated. The organic layer was concentrated under vacuum and the residue was purified by preparative high performance liquid chromatography (HPLC) to afford the target product 5-bromo-2-iodoisopropylbenzene as a white solid (189 mg, 10% yield). [0257] 1H NMR (500 MHz, DMSO-d6) δ 1.18 (d, J = 6.8 Hz, 6H), 3.07 (qn, J = 6.8 Hz, 1H), 7.15 (dd, J = 8.4,2.5 Hz, 1H), 7.46 (d, J = 2.4 Hz, 1H), 7.75 (d, J = 8.4 Hz, 1H). 2D NOE (500 MHz, DMSO-d6): cross peak between δ 1.18 and 7.46.
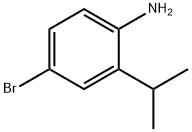
81090-34-8
43 suppliers
$140.00/500mg

1147014-97-8
41 suppliers
$31.00/250mg
Yield:1147014-97-8 73%
Reaction Conditions:
Stage #1: 4-bromo-2-isopropyl-anilinewith hydrogenchloride;sodium nitrite in water at 0; for 1 h;
Stage #2: with potassium iodide in water at 0 - 20; for 16 h;
References:
Trobe, Melanie;Blesl, Julia;Vareka, Martin;Schreiner, Till;Breinbauer, Rolf [European Journal of Organic Chemistry,2022,vol. 2022,# 17,art. no. E202101279] Location in patent:supporting information

19099-54-8
103 suppliers
$5.00/1g

1147014-97-8
41 suppliers
$31.00/250mg
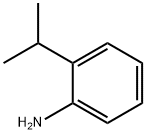
643-28-7
190 suppliers
$7.00/5g

1147014-97-8
41 suppliers
$31.00/250mg