
4-broMo-1-Methyl-1H-iMidazole-2-carbaldehyde synthesis
- Product Name:4-broMo-1-Methyl-1H-iMidazole-2-carbaldehyde
- CAS Number:79326-91-3
- Molecular formula:C5H5BrN2O
- Molecular Weight:189.01
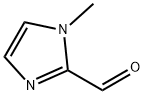
13750-81-7
242 suppliers
$6.00/250mg

79326-91-3
19 suppliers
inquiry
Yield: 40%
Reaction Conditions:
with N-Bromosuccinimide in N,N-dimethyl-formamide at 20; for 144 h;
Steps:
-Bromo-1 -methyl-1 H-imidazole-2-carbaldehydeA solution of 1 -methy-1 H-imidazole-2-carbaldehyde (10 g, 90 mmol) in deoxygenated DMF (300 mL) was treated with N-bromosuccinimide(17.8 g, 100 mmol) and the resulting solution stirred at RT for 6 days. Water (750 mL) was added and the resulting mixture was extracted with EtOAc (4 X 200 mL) the combined extracts were dried over Na2SO4, filtered and the volatiles removed in vacuo. The residue was purified by column chromatography on silica gel (n-heptane/EtOAc = 4/1 ) to yield the title compound (7.33 g, 40%) as a white solid. LC-MS: m/z = 191 .2 (MH+), tR = 0.48 minutes, method A. 1H NMR (600 MHz, DMSO-c/6): δ 9.62 (s, 1 H), 7.80 (s, 1 H), 3.92 (s, 3H).
References:
H. LUNDBECK A/S;PÜSCHL, Ask;NIELSEN, Jacob;KEHLER, Jan;KILBURN, John, Paul;MARIGO, Mauro;LANGGÅRD, Morten WO2011/72694, 2011, A1 Location in patent:Page/Page column 42-43
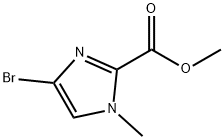
864076-05-1
64 suppliers
$82.00/1g

79326-91-3
19 suppliers
inquiry