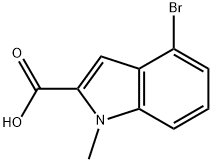
4-bromo-1-methyl-1H-indole-2-carboxylic acid synthesis
- Product Name:4-bromo-1-methyl-1H-indole-2-carboxylic acid
- CAS Number:880349-08-6
- Molecular formula:C10H8BrNO2
- Molecular Weight:254.08

167478-92-4
2 suppliers
inquiry

880349-08-6
7 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 4-bromo-1-methyl-1H-indole-2-carboxylic acid methyl esterwith methanol;lithium hydroxide;water in tetrahydrofuran; for 16 h;
Stage #2: with hydrogenchloride;water in ethyl acetate;
Steps:
C1
Intermediate Cl : 4-Bromo-l -methyl- lH-indole-2-carboxylic acidTo a solution of 4-bromo-lH-indole-2-carboxylic acid (514 mg, 2.14 mmol) in DMF (16 mL), dimethyl carbonate (4.5 mL, 53.4 mmol) and DABCO (25 mg, 0.214 mmol) was added, and the solution was heated to 12O0C for 7 hours. The reaction was diluted with EtOAc, and the organics were washed with H2O (2x), IN HCl (Ix), and brine (Ix). The organics were dried over Na2SO4, filtered, concentrated, and the resulting residue was purified on SiO2 (gradient elution, 15-40% EtOAc/hexanes) to yield the intermediate ester as a white solid. MeOH (3 mL), H2O (1.5 mL) and LiOH monohydrate (3 eq.) were added to a solution of the ester in THF (3 mL), and left to stir for 16 hours. The reaction mixture was concentrated, and the residue was partitioned between EtOAc and IN HCl, and extracted with EtOAc (2x). The organics were combined, washed with brine (Ix), dried over Na2SO4, filtered, and concentrated to yield the title compound as a white solid. LRMS (M+H)+ Calcd. = 254; found 254.
References:
WO2008/57209,2008,A1 Location in patent:Page/Page column 51