
4-Bromo-1H-benzo[cd]indol-2-one synthesis
- Product Name:4-Bromo-1H-benzo[cd]indol-2-one
- CAS Number:37960-55-7
- Molecular formula:C11H6BrNO
- Molecular Weight:248.08
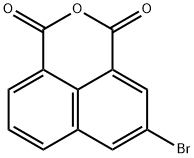
24050-49-5
103 suppliers
$63.79/250mg
![4-Bromo-1H-benzo[cd]indol-2-one](/CAS/20210111/GIF/37960-55-7.gif)
37960-55-7
6 suppliers
inquiry
Yield:37960-55-7 31%
Reaction Conditions:
Stage #1: 5-bromo-1H,3H-benzo[de]isochromene-1,3-dionewith pyridine;hydroxyamino hydrochloride for 2 h;Reflux;
Stage #2: with 4-methylbenzene-1-sulfonyl chloride for 2 h;Reflux;
Stage #3: with hydrogenchloride;sodium hydroxide in ethanol;lithium hydroxide monohydrate; for 3 h;
Steps:
5.1.1.2. 4-bromobenzo[cd]indol-2(1H)-one (19).
To a solution of 5-bromo-1H,3H-benzo[de]isochromene-1,3-dione 18 (10 g, 36.2 mmol, 1equiv) and hydroxylamine hydrochloride (2.5 g, 36.2 mmol, 1 equiv) inpyridine (70 mL) was conducted under reflux for 2 h, followed bycooling to 80 °C. Then ptoluenesulfonyl chloride (13.8 g, 72.4 mmol, 2equiv) was added to the reaction system. After addition, thetemperature was raised and the reaction was carried out under refluxfor 2 h, followed by cooling. The reaction mixture was poured into0.40 L of water and stirred to precipitate crystals, which were collectedby filtration. The crystals were transferred to a beaker and washedsuccessively with 0.5 L of a NaHCO3 aqueous solution and 0.5 L ofwater, followed by filtration. The crystals were washed with water and dried to give an intermediate for further reaction. The whole amount ofthe intermediate, 40 mL of ethanol and 50 mL of water were put in areactor and stirred. Then 130 mL of a 1.4 mol/L aqueous solution ofNaOH was added dropwise to the mixture. Thereafter, the mixture washeated to refluxing temperature, at which the reaction was carried outfor 3 h while distilling off ethanol. After completion of the reaction, thereaction mixture was cooled to 75 °C, and 60 mL of concentrated HClwas added dropwise. In the meantime, crystals precipitated at 60 °C.After completion of the dropwise addition, the mixture was furthercooled. The precipitated crystals were collected by filtration, washedwith ion-exchanged water, and dried to give the title compound 19 aswhite solid (2.76 g, 11.22 mmol, 31% yield). [M+H]+: 248.1. 1H NMR(300 MHz, DMSO-d6) δ 10.92 (s, 1H), 8.44 (s, 1H), 8.10 (s, 1H), 7.58 (d,J=3.5 Hz, 2H), 7.01 (dd, J=5.9, 1.8 Hz, 1H).
References:
Cao, Peng;Chen, Haifang;Chen, Yadong;Cui, Yong;Jiang, Fei;Li, Hongmei;Li, Huili;Lu, Tao;Ma, Yu;Wei, Qingyun [Bioorganic and medicinal chemistry,2019]
![Benz[cd]indol-2(1H)-one, 4-amino-](/CAS/20210111/GIF/67522-42-3.gif)
67522-42-3
3 suppliers
inquiry
![4-Bromo-1H-benzo[cd]indol-2-one](/CAS/20210111/GIF/37960-55-7.gif)
37960-55-7
6 suppliers
inquiry
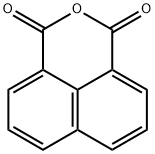
81-84-5
231 suppliers
$13.00/25g
![4-Bromo-1H-benzo[cd]indol-2-one](/CAS/20210111/GIF/37960-55-7.gif)
37960-55-7
6 suppliers
inquiry
![Benz[cd]indol-2(1H)-one, 7-bromo-](/CAS/20210305/GIF/24950-36-5.gif)