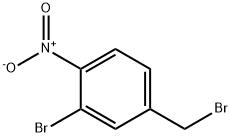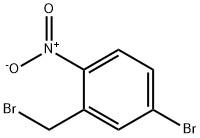
4-bromo-2-(bromomethyl)-1-nitrobenzene synthesis
- Product Name:4-bromo-2-(bromomethyl)-1-nitrobenzene
- CAS Number:35287-42-4
- Molecular formula:C7H5Br2NO2
- Molecular Weight:294.93
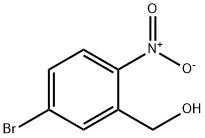
1241894-37-0

35287-42-4
To a stirred solution of (5-bromo-2-nitrophenyl)methanol (2.8 g, 12.06 mmol) in a solvent mixture of dichloromethane (DCM) and acetonitrile (MeCN) (1:1, 100 mL) was added sequentially at 0 °C carbon tetrabromide (CBr4) (5.2 g, 15.68 mmol) and triphenylphosphine (PPh3) (4.1 g. 15.68 mmol). The reaction mixture was stirred at room temperature for 16 hours. Upon completion of the reaction, the mixture was concentrated under reduced pressure and the residue was purified by fast column chromatography on silica gel (petroleum ether (PE)/ethyl acetate (EA) = 50:1 to 10:1 gradient elution) to afford the target product 4-bromo-2-(bromomethyl)-1-nitrobenzene (1.1 g, 31% yield).

1241894-37-0
53 suppliers
inquiry

35287-42-4
64 suppliers
inquiry
Yield: 31%
Reaction Conditions:
with carbon tetrabromide;triphenylphosphine in dichloromethane;acetonitrile at 0 - 20; for 16 h;
Steps:
12; 13 4-Bromo-2-(bromomethyl)-1-nitrobenzene
To a stirred solution of (5-bromo-2-nitrophenyl) methanol (2.8 g, 12.06 mmol) in a mixture of DCM/MeCN (1:1, 100 mL) at 0 °C, CBr4 (5.2 g, 15.68 mmol) and PPh3 (4.1 g, 15.68 mmol) were added and the resulting mixture was stirred at RT for 16 h. The mixture was concentrated in vacuo and the residue was purified by flash column chromatography on silica gel (PE/EA = 50:1 to 10:1) to afford the desired product (1.1 g, 31% yield).
References:
ARAXES PHARMA LLC;WU, Tao;WANG, Yi;LI, Liansheng;FENG, Jun;LIU, Yuan;REN, Pingda;LIU, Yi WO2018/68017, 2018, A1 Location in patent:Page/Page column 154; 155; 156; 157
823-78-9
286 suppliers
$8.00/10g

35287-42-4
64 suppliers
inquiry
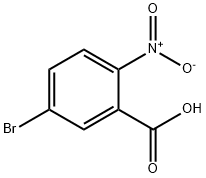
6950-43-2
114 suppliers
$6.00/250mg

35287-42-4
64 suppliers
inquiry
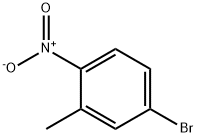
52414-98-9
126 suppliers
$18.00/1g

35287-42-4
64 suppliers
inquiry