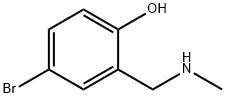
4-BROMO-2-[(ETHYLAMINO)METHYL]PHENOL synthesis
- Product Name:4-BROMO-2-[(ETHYLAMINO)METHYL]PHENOL
- CAS Number:157729-23-2
- Molecular formula:C8H10BrNO
- Molecular Weight:216.08
22532-62-3
325 suppliers
$5.00/250mg

74-89-5
0 suppliers
$13.44/25ML
![4-BROMO-2-[(ETHYLAMINO)METHYL]PHENOL](/CAS/GIF/157729-23-2.gif)
157729-23-2
29 suppliers
$45.00/50mg
Yield:157729-23-2 35%
Reaction Conditions:
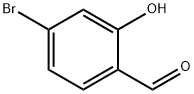
Stage #1: 4-bromosalicylaldehyde;methylamine for 3 h;Dean-Stark;Reflux;
Stage #2: with sodium tetrahydroborate at 20; for 4 h;Dean-Stark;
Steps:
4-Bromo-2-((methylamino)methyl)phenol (3).
To an ethanolicsolution (100 mL) of 4-bromo-salicylaldehyde (5 mL,46.7mmol), methylamine (8.06 mL, 93.4mmol) was added.The mixture was heated under reflux in a round-bottom flaskfitted with a Dean-Stark trap to remove the water produced during the reaction. After heating for 3 h, the solution isleft at room temperature and then sodium borohydride(0.90 g, 23.3mmol) was added. The solution was stirred for2 h before adding a second fraction of sodium borohydride(0.90 g, 23.3mmol). After stirring for 2 h, the solution wascooled in an ice bath and acidified to pH 2. The solventwas evaporated and the residue was extracted with distilledwater/diethyl ether (3 × 20 mL). The combined aqueousphases were adjusted to pH 11 by addition of aqueouspotassium hydroxide and extracted with dichloromethane(3 × 30 mL). The combined organic phases were dried withanhydrous sodium sulphate, and the solvent was evaporated.The solid was dried in vacuo. Yield 35%; Elemental analysiscalculated for C8H10BrNO (263.29): C 44.4, H 4.6, N 6.5;found: C 44.2, H 4.4, N 6.5. 1H NMR (300MHz, CDCl3): δ7.19-6.63 (m, 3H), 4.8-4.2 (a, 1H), 3.86 (s, 2H), 2.40 (s, 3H).ES-MS: m/z 264. IR: ν(O-H) 3014, ](N-H) 2736 cm-1.
References:
Pérez-Otero, Yolanda;Fernández-García, M. Isabel;Gómez-Fórneas, Esther;González-Riopedre, Gustavo;Maneiro, Marcelino [Journal of Chemistry,2015,vol. 2015,art. no. 963152]