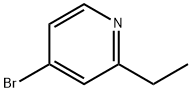
4-bromo-2-ethylpyridine synthesis
- Product Name:4-bromo-2-ethylpyridine
- CAS Number:156761-88-5
- Molecular formula:C7H8BrN
- Molecular Weight:186.05
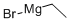
925-90-6
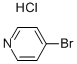
19524-06-2

156761-88-5
The general procedure for the synthesis of 4-bromo-2-ethylpyridine from ethylmagnesium bromide and 4-bromopyridine hydrochloride was as follows: the preparation was carried out according to the method described in Journal of Organic Chemistry, 1985, Vol. 50, No. 22, pp. 4410-4411. First, a THF (100 mL) slurry of 4-bromopyridine hydrochloride (4.17 g, 0.021 mol) was cooled to -78°C under nitrogen protection. Subsequently, ethylmagnesium bromide (15.7 mL, 3.0 M ether solution, 2.2 eq.) was added slowly dropwise via syringe. The reaction mixture was stirred at -78 °C for 10 min before removing the ice bath and allowing it to warm naturally to room temperature. Upon completion of the reaction, it was quenched with 20% aqueous ammonium chloride solution and diluted with ether. The organic phase was washed sequentially with water, 1N HCl aqueous solution and saturated sodium chloride aqueous solution. The organic layer was dried over magnesium sulfate, filtered and concentrated in vacuum. The residue was redissolved in toluene (100 mL) and tetrachloro-1,2-benzoquinone (5.8 g, 1.1 eq.) pre-dissolved in acetic acid (50 mL) was added under nitrogen protection. The reaction mixture was stirred overnight at room temperature. Subsequently, the pH was adjusted to basic with 1N NaOH aqueous solution and extracted with ethyl acetate. The organic phase was acidified with 1N HCl aqueous solution and extracted again with ethyl acetate. The organic phases were combined, washed with saturated aqueous sodium chloride solution, dried over magnesium sulfate, filtered and concentrated in vacuo to give a brown residue (2.64 g, 66% yield).LCMS (ES) analysis showed m/z 188 (M+1, bromide mode).
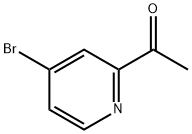
1060805-69-7
86 suppliers
$93.59/250mg

156761-88-5
64 suppliers
$55.00/10mg
Yield:156761-88-5 91.4%
Reaction Conditions:
with aluminum (III) chloride;sodium tetrahydroborate in tetrahydrofuran at -5 - 65; for 12 h;Inert atmosphere;Temperature;
Steps:
1; 4-5; 1-2 Synthesis of 2-ethyl-4-bromopyridine
Add 50.0g (0.25mol, 1eq) of 1-(4-bromopyridin-2-yl)ethanone and 400g of tetrahydrofuran to a 1000ml four-necked reaction flask, start stirring, The reaction flask was replaced with nitrogen for protection, the temperature was reduced to -5°C, 14.2g (0.375mol, 1.5eq) of sodium borohydride and 66.7g (0.5mol, 2eq) of aluminum trichloride were added, After the addition, the temperature is slowly raised to 0°C and the reaction is stirred for 2h, and then heated to reflux (about 65°C) for 10h (detection of raw materials The solvent was removed under reduced pressure, and 300 g of water was slowly added dropwise to the residue under the protection of nitrogen, and the temperature was controlled not to exceed 35°C in an ice water bath. After dripping, it was stirred for 2h in an ice-water bath, extracted with chloroform 300g×2, combined the organic phases, and dissolved, the residue was 46.4g, recrystallized with ethanol 139.2g to obtain 42.5g of 2-ethyl-4-bromopyridine. The purity measured by HPLC was 98.4%, and the yield was 91.4%.
References:
CN112194620,2021,A Location in patent:Paragraph 0016; 0020; 0026-0027; 0029-0030
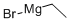
925-90-6
349 suppliers
$12.00/5ml

19524-06-2
485 suppliers
$6.00/5g

156761-88-5
64 suppliers
$55.00/10mg

101860-96-2
5 suppliers
inquiry

156761-88-5
64 suppliers
$55.00/10mg
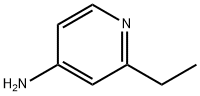
50826-64-7
40 suppliers
$45.00/50mg

156761-88-5
64 suppliers
$55.00/10mg
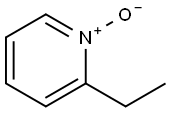
4833-24-3
20 suppliers
$45.00/100mg

156761-88-5
64 suppliers
$55.00/10mg