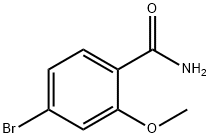
4-BroMo-2-MethoxybenzaMide synthesis
- Product Name:4-BroMo-2-MethoxybenzaMide
- CAS Number:812667-44-0
- Molecular formula:C8H8BrNO2
- Molecular Weight:230.06
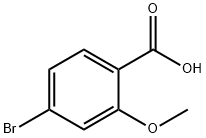
72135-36-5
142 suppliers
$6.00/1g

812667-44-0
17 suppliers
$100.00/250mg
Yield:812667-44-0 89%
Reaction Conditions:
Stage #1: 4-bromo-2-methoxybenzoic acidwith 1,1′-carbonyldiimidazole in tetrahydrofuran at 20; for 1 h;
Stage #2: with ammonium hydroxide in tetrahydrofuran at 20; for 2 h;
Steps:
4.1.1.5. Procedure E: General procedure for the synthesis of the primary amides (K, a-v) from the respective carboxylic acids
General procedure: A mixture of theappropriate carboxylic acid (3 mmol, 1 equiv) and 1,1′ -carbonyldiimidazole(4.5 mmol, 1.5 equiv) in 30 ml anhydrous THF was stirredat room temperature for 1 h. This was followed by dropwise addition of15 ml 25% aqueous ammonia solution and stirring at room temperaturefor 2 h. Afterward, the mixture was diluted with water and extractedwith ethyl acetate (3 × 30 ml). To get rid of trace CDI and imidazole, thecombined organic layers were washed once either with 20 ml of 1 N HClor with 20 ml of 0.05 M potassium phosphate buffer pH 5.8 in case ofamides having basic heterocyclic rings (s-v). The organic fraction wasthen dried over anhydrous MgSO4 and evaporated under reduced pressureto give the pure primary amides in different yields, used directly innext steps without further purification.
References:
El-Gamil, Dalia S.;ElHady, Ahmed K.;Chen, Po-Jen;Hwang, Tsong-Long;Abadi, Ashraf H.;Abdel-Halim, Mohammad;Engel, Matthias [European Journal of Medicinal Chemistry,2022,vol. 238,art. no. 114411]
330793-38-9
149 suppliers
inquiry

812667-44-0
17 suppliers
$100.00/250mg
139102-34-4
147 suppliers
$5.00/250mg

812667-44-0
17 suppliers
$100.00/250mg
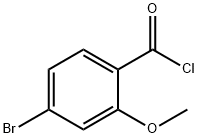
5213-16-1
8 suppliers
inquiry

812667-44-0
17 suppliers
$100.00/250mg