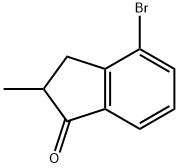
4-Bromo-2-methyl-1-indanone synthesis
- Product Name:4-Bromo-2-methyl-1-indanone
- CAS Number:174702-59-1
- Molecular formula:C10H9BrO
- Molecular Weight:225.08
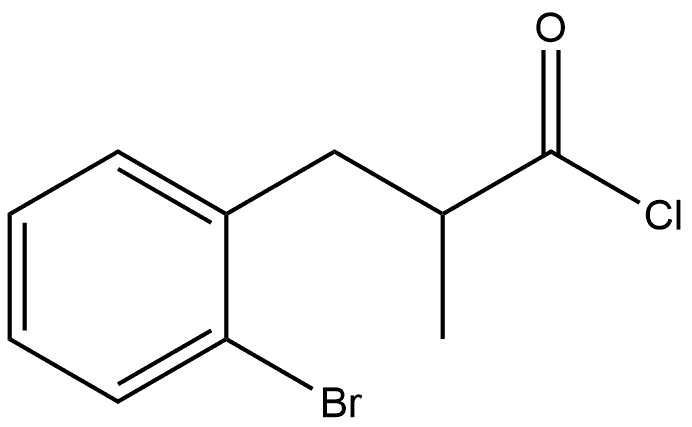
869063-66-1

174702-59-1
General procedure for the synthesis of 4-bromo-2-methyl-2,3-dihydro-1H-inden-1-one from the compound (CAS: 869063-66-1): a mixture of starting acid (unspecified amount) and 160 mL of thionyl chloride (SOCl2) was stirred for 24 hours at room temperature. Subsequently, the excess thionyl chloride was removed by distillation. Under vigorous stirring, crude 3-(2-bromophenyl)-2-methylpropionyl chloride (dissolved in 270 mL of dichloromethane) was added drop-wise to 1350 mL of dichloromethane suspension containing 136 g (1.02 mol) of aluminum trichloride (AlCl3), keeping the reaction temperature at 0 °C for 1 hour. After that, the reaction mixture was refluxed for 3 h, cooled to room temperature and poured into 500 mL of ice water. The organic layer was separated and the aqueous layer was extracted with methyl tert-butyl ether (3 x 300 mL). The organic phases were combined and dried over potassium carbonate (K2CO3) and subsequently evaporated to dryness. Purification by fractional distillation afforded 4-bromo-2-methyl-1-indanone with a boiling point of 131-134 °C/2 mmHg in a yield of 125.5 g (75% yield) as a colorless solid. Elemental analysis (calculated value C10H9BrO): C, 53.36; H, 4.03. measured value: C, 53.19; H, 3.98. 1H NMR (300 MHz, CDCl3) δ: 7.76 (d, J = 7.6 Hz, 1H, 7-H), 7.71 (d, J = 7.6 Hz, 1H, 5-H), 7.28 (t, J = 7.6 Hz, 1H, 5-H), 7.28 (t, J = 7.6 Hz, 1H, 5-H). 7.6 Hz, 1H, 6-H), 3.36 (dd, J = 17.5 Hz, J = 7.6 Hz, 1H, 3-H), 2.70-2.82 (m, 1H, 2-H), 2.67 (dd, J = 17.5 Hz, J = 3.8 Hz, 1H, 3'-H), 1.34 (d, J = 7.3 Hz, 3H, 2-Me).13C NMR (75 MHz, CDCl3) δ: 208.3, 152.9, 138.2, 137.2, 129.0, 122.6, 122.0, 41.8, 35.7, 16.0.

869063-66-1
0 suppliers
inquiry

174702-59-1
88 suppliers
$45.00/1 g
Yield: 125.5 g (75%)
Reaction Conditions:
with thionyl chloride;AlCl3 in dichloromethane
Steps:
1 4-bromo-2-methyl-1-indanone via 3-(2-bromophenyl)-2-methylpropionyl chloride via 3-(2-bromophenyl)-2-methylpropionic acid
A mixture of this acid and 160 ml of SOCl2 was stirred for 24 hours at ambient temperature. Thionyl chloride was distilled off. The crude 3-(2-bromophenyl)-2-methylpropionyl chloride dissolved in 270 ml of CH2Cl2 was added dropwise with vigorous stirring to a suspension of 136 g (1.02 mol) of AlCl3 in 1350 ml of CH2Cl2 for 1 hour at 0° C. Then, this mixture was refluxed for 3 hours, cooled to ambient temperature, and poured on 500 cm3 of ice. The organic layer was separated. The aqueous layer was extracted with 3*300 ml of methyl-tert-butyl ether. The combined extract was dried over K2CO3 and evaporated to dryness. Fractional distillation gave 4-bromo-2-methyl-1-indanone, b.p. 131-134° C./2 mm Hg. Yield, 125.5 g (75%) of a colorless solid. Anal. calc. for C10H9BrO: C, 53.36; H, 4.03. Found: C, 53.19; H, 3.98. 1H NMR (300 MHz, CDCl3): δ 7.76 (d, J=7.6 Hz, 1H, 7-H), 7.71 (d, J=7.6 Hz, 1H, 5-H), 7.28 (t, J=7.6 Hz, 1H, 6-H), 3.36 (dd, J=17.5 Hz, J=7.6 Hz, 1H, 3-H), 2.70-2.82 (m, 1H, 2-H), 2.67 (dd, J=17.5 Hz, J=3.8 Hz, 1H, 3'-H), 1.34 (d, J=7.3 Hz, 3H, 2-Me). 13C NMR (75 MHz, CDCl3): δ 208.3, 152.9, 138.2, 137.2, 129.0, 122.6, 122.0, 41.8, 35.7, 16.0.
References:
Voskoboynikov, Alexander Z.;Izmer, Vyatcheslav V.;Asachenko, Andrey F.;Nikulin, Mikhail V.;Ryabov, Alexey N.;Lebedev, Artyom Y.;Coker, Catalina L.;Canich, Jo Ann M. US2007/135597, 2007, A1
19829-31-3
165 suppliers
$16.00/1g

174702-59-1
88 suppliers
$45.00/1 g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

174702-59-1
88 suppliers
$45.00/1 g
6630-33-7
426 suppliers
$9.00/10g

174702-59-1
88 suppliers
$45.00/1 g