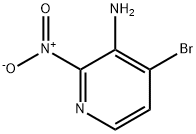
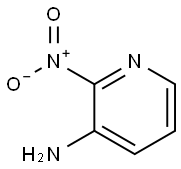
4-bromo-2-nitropyridin-3-amine synthesis
- Product Name:4-bromo-2-nitropyridin-3-amine
- CAS Number:1305317-30-9
- Molecular formula:C5H4BrN3O2
- Molecular Weight:218.01
13269-19-7
315 suppliers
$6.00/1g

1305317-30-9
36 suppliers
inquiry
Yield:1305317-30-9 96%
Reaction Conditions:
Stage #1: 3-amino-2-nitropyridinewith potassium acetate;acetic acid at 20; for 1 h;
Stage #2: with bromine at 20; for 16 h;
Steps:
294.A; 295.A Part A. 4-bromo-2-nitropyridin-3-amine
To a solution of 2-nitropyridin-3-amine (4 g, 28.8 mmol) in AcOH (40 mL) was added potassium acetate (2.82 g, 28.8 mmol) and the mixture stirred for 1 h at room temperature. Br2 (1.481 mL, 28.8 mmol) was added slowly to the reactionmixture and the mixture stirred at room temperature for 16 h. The solid formed wascollected by vacuum filtration, washed with diethyl ether (2x10 mL) and dried underhigh vacuum to afford 4-bromo-2-nitropyridin-3-amine (6 g, 27.5 mmol, 96% yield) as a yellow solid. LCMS (ESI)m/e 218.0 [(M+H), calcd for C5H5BrN3O2 218.01; LC/MS retention time (method B): tR = 0.61 mm.
References:
WO2017/59080,2017,A1 Location in patent:Page/Page column 111; 114