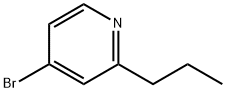
4-broMo-2-propylpyridine synthesis
- Product Name:4-broMo-2-propylpyridine
- CAS Number:98420-99-6
- Molecular formula:C8H10BrN
- Molecular Weight:200.08
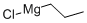
2234-82-4
109 suppliers
$63.95/100ml
19524-06-2
483 suppliers
$6.00/5g

98420-99-6
22 suppliers
$208.00/250mg
Yield:98420-99-6 63%
Reaction Conditions:
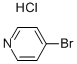
Stage #1: 1-propylmagnesium chloride;4-bromopyridine hydrochloridewith phenyl chloroformate in tetrahydrofuran;diethyl ether at -78; for 0.333333 h;Inert atmosphere;
Stage #2: with chloranil;acetic acid in toluene at 20; for 16 h;
Steps:
28.1 preparation of intermediate 4-bromo-2-propylpyridine
Under a nitrogen atmosphere, a suspension of 4-bromopyridine hydrochloride (1 .0 g, 5.14 mmol) in anhydrous tetrahydrofuran (20 mL) was cooled to -78°C. Propylmagnesium chloride 2.0M in diethyl ether (5.66 mL, 1 1.31 mmol) was dropwise added, followed by phenyl chloroformate (0.77 mL, 6.17 mmol) 5 minutes later. The mixture was stirred at -78°C for 15 minutes, then allowed to reach 10°C to be quenched by adding a saturated solution of ammonium chloride (20 mL). Diethyl ether (10 mL) was added, and the organic layer was successively washed with 1 N hydrochloric acid (20 mL), water (20 mL) and brine (20 mL). The organic layer was dried over sodium sulfate and concentrated in vacuo. The residue was dissolved in anhydrous toluene (20 mL). To this solution was dropwise added a solution of o- chloranil (1 .39 g, 5.66 mmol) in glacial acetic acid (15 mL). The mixture was stirred at room temperature for 16 hours, then cooled to 0°C, and basified with 1 N sodium hydroxide until pH 10. The mixture was extracted with ethyl acetate (2 x 10 mL). The organic layer was extracted with 1 N hydrochloric acid (2 x 10 mL). The acidic aqueous layer was washed with ethyl acetate (2 x 10 mL), basified with 1 N sodium hydroxide until pH 10, and extracted with ethyl acetate (2 x 10 mL). The combined organic layers were dried over sodium sulfate and concentrated in vacuo. The residue was purified by flash chromatography on silica gel (cyclohexane/ethyl acetate 100/0 to 95/5) to provide 4-bromo-2-propylpyridine (28a) (649 mg, 3.24 mmol, 63%) as a colorless oil. 1H NMR (400 MHz, CDCI3) δ 0.97 (t, J = 7.4 Hz, 3H), 1.45 (sext, J = 7.4 Hz, 2H), 2.74 (t, J = 7.4 Hz, 2H), 7.27 (dd, J = 5.3 Hz, J = 1.8 Hz, 1 H), 7.33 (d, J = 1.8 Hz, 1 H), 8.33 (d, J = 5.3 Hz, 1 H).
References:
WO2014/57103,2014,A1 Location in patent:Page/Page column 143

19524-06-2
483 suppliers
$6.00/5g

98420-99-6
22 suppliers
$208.00/250mg