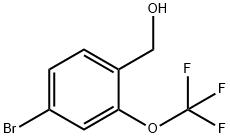
(4-Bromo-2-(trifluoromethoxy)phenyl)methanol synthesis
- Product Name:(4-Bromo-2-(trifluoromethoxy)phenyl)methanol
- CAS Number:220996-81-6
- Molecular formula:C8H6BrF3O2
- Molecular Weight:271.03
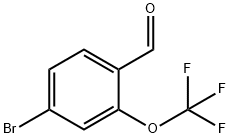
220996-80-5

220996-81-6
[Step 2] Synthesis of (4-bromo-2-(trifluoromethoxy)phenyl)methanol: To a solution of 4-bromo-2-(trifluoromethoxy)benzaldehyde (16 g, 59 mmol) in methanol (0.23 L) was added sodium borohydride (2.4 g, 63 mmol) in batches at -10 °C. The reaction solution was stirred at -10 °C for 10 min before acetone (10 mL) and 1N hydrochloric acid (10 mL) were added sequentially to quench the reaction. The reaction mixture was concentrated under reduced pressure and water was added to the residue and extracted with ethyl acetate. The organic layers were combined, dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography (elution gradient: hexane/ethyl acetate = 50:1 → 1:1) to afford 15 g (91% yield) of the title compound (4-bromo-2-(trifluoromethoxy)phenyl)methanol (hereinafter referred to as Reference Example Compound 2).

220996-80-5
125 suppliers
$7.00/250mg

220996-81-6
89 suppliers
$22.00/250mg
Yield:220996-81-6 95%
Reaction Conditions:
with sodium tetrahydroborate in methanol;
References:
Williams, Peter D.;Bock, Mark G.;Evans, Ben E.;Freidinger, Roger M.;Gallicchio, Steven N.;Guidotti, Maribeth T.;Jacobson, Marlene A.;Michelle S, Kuo;Levy, Michelle R.;Edward V, Lis;Michelson, Stuart R.;Pawluczyk, Joseph M.;Perlow, Debra S.;Pettibone, Douglas J.;Quigley, Amy G.;Reiss, Duane R.;Salvatore, Christopher;Stauffer, Kenneth J.;Woyden, Carla J. [Bioorganic and Medicinal Chemistry Letters,1999,vol. 9,# 9,p. 1311 - 1316]