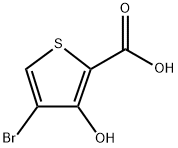
4-Bromo-3-hydroxythiophene-2-carboxylic acid synthesis
- Product Name:4-Bromo-3-hydroxythiophene-2-carboxylic acid
- CAS Number:634187-30-7
- Molecular formula:C5H3BrO3S
- Molecular Weight:223.04
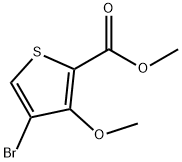
110545-67-0
57 suppliers
$49.47/250mgs:

634187-30-7
2 suppliers
inquiry
Yield:634187-30-7 100%
Reaction Conditions:
with hydrogenchloride;sodium hydroxide in tetrahydrofuran at 20; pH=2; for 24 h;
Steps:
13.19 Preparative example 13.19
Methyl-3-methoxy-4-bromo-2-thiophenecarboxylate (13.7 g), available from step A, was dissolved in 75 mL of THF, and added with a 1.0 M sodium hydroxide aqueous solution (65 mL, 65.0 mmol). The mixture was stirred at room temperature for 24 h. A 1.0 M hydrogen chloride aqueous solution was added dropwise to the mixture until pH was approximately 2. The acidic mixture was extracted with CH2Cl2 (100 mL?2, 50 mL). The combined organic extracts were washed with brine (40 mL), dried with Na2SO4, and concentrated under reduced pressure to a solid, 10.0 g (100%, over two steps) of 3-methoxy-4-bromo-2-thiophenecarboxylic acid (MH+=237.0).
References:
US2004/106794,2004,A1 Location in patent:Page 93
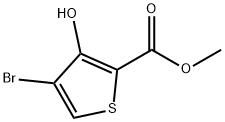
95201-93-7
94 suppliers
$24.00/100mg

634187-30-7
2 suppliers
inquiry