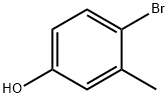
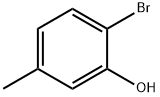
4-Bromo-3-methylphenol synthesis
- Product Name:4-Bromo-3-methylphenol
- CAS Number:14472-14-1
- Molecular formula:C7H7BrO
- Molecular Weight:187.03
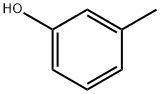
108-39-4

14472-14-1
General procedure for the synthesis of 4-bromo-3-methylphenol from m-cresol: (a) Preparation of 4-bromo-3-methylphenol: 80.0 g (740 mmol) of m-cresol and 400 mL of glacial acetic acid were added to a 2 L three-neck flask under nitrogen protection. The reaction mixture was cooled to 15 °C, 38 mL (742 mmol) of bromine was added slowly dropwise, and the reaction was stirred for 3 h at 15 °C maintaining the temperature. After completion of the reaction, the reaction solution was poured into 1 L of water and extracted with ether. The organic phase was separated and washed with water to neutral pH and subsequently dried with magnesium sulfate. The solvent was removed by evaporation under reduced pressure and the resulting residue was ground in heptane, filtered and dried to give 70 g (50% yield) of a white powdery product with a melting point of 55-56 °C.

108-39-4
512 suppliers
$13.00/25g

14472-14-1
269 suppliers
$10.00/5g
Yield:14472-14-1 96%
Reaction Conditions:
with o-xylylene bis(triethylammonium tribromide) in acetonitrile at 20; for 0.0833333 h;regioselective reaction;
Steps:
3.3. General Procedure for Bromination of AromaticCompounds
General procedure: To a magnetic solution of aromatic compound (1 mmol)in acetonitrile (5 mL), OXBTEATB (0.233 g, 0.5 mmol) wasadded and stirred at room temperature for the appropriatetime (Table 1). The reaction was monitored by TLC (eluent:n-hexane/ethyl acetate: 5/1). The reaction mixture was transferredinto a separatory funnel after filtration of OXBTEABand was extracted with water (15 mL) and dichloromethane(20 mL). The organic layer was dried over anhydrousNa2SO4, and the solvent was concentrated in a rotary evaporator.The crude product was purified by passing it over acolumn of silica gel using a mixture of n-hexane and ethylacetate as the eluent. In order to regenerate the reagent, whitesolid was treated with liquid bromine. All the product structureswere confirmed by comparison of melting point or 1HNMR spectra with ones reported in the literature [29a-29e].
References:
Hemati, Roya;Shahvelayati, Ashraf S.;Yadollahzadeh, Khadijeh [Letters in Organic Chemistry,2018,vol. 15,# 8,p. 682 - 687]

108-39-4
512 suppliers
$13.00/25g

14472-14-1
269 suppliers
$10.00/5g
553-97-9
206 suppliers
$5.00/5g
1193-18-6
182 suppliers
$6.00/1g

14472-14-1
269 suppliers
$10.00/5g

1193-18-6
182 suppliers
$6.00/1g

14472-14-1
269 suppliers
$10.00/5g

108-39-4
512 suppliers
$13.00/25g

108-39-4
512 suppliers
$13.00/25g

14472-14-1
269 suppliers
$10.00/5g
14847-51-9
129 suppliers
$9.00/100mg