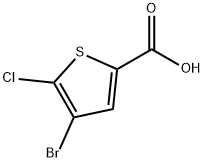
4-broMo-5-chlorothiophene-2-carboxylic acid synthesis
- Product Name:4-broMo-5-chlorothiophene-2-carboxylic acid
- CAS Number:60729-37-5
- Molecular formula:C5H2BrClO2S
- Molecular Weight:241.49
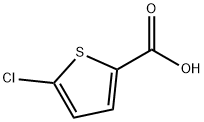
24065-33-6

60729-37-5
The general procedure for the synthesis of 4-bromo-5-chlorothiophene-2-carboxylic acid from 2-chlorothiophene-5-carboxylic acid was as follows: a solution of bromine (634 μL, 12.3 mmol) in acetic acid (2.5 mL) was added slowly and dropwise to an acetic acid solution (25 mL) containing 5-chloro-2-thiophenecarboxylic acid (2 g, 12.3 mmol) and ferric chloride (399 mg, 2.50 mmol). mL) at 25 °C. Subsequently, the reaction mixture was heated to reflux during which bromine (634 μL, 12.3 mmol) and ferric chloride (399 mg, 2.50 mmol) were added in portions. After the reaction lasted for 7 days, the reaction solution was poured into ice water, the precipitate precipitated was filtered and washed with cold water to finally obtain 4-bromo-5-chlorothiophene-2-carboxylic acid (3 g, quantitative yield) as a yellow powder. The product was analyzed by liquid chromatography-mass spectrometry (LC-MS) and showed m/z 242 ([M+H]+).
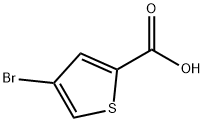
16694-18-1
212 suppliers
$6.00/1g

60729-37-5
44 suppliers
$79.00/100mg
Yield: 79%
Reaction Conditions:
with N-chloro-succinimide in N,N-dimethyl-formamide at 50; for 10 h;
Steps:
89.a
To a solution of 4-bromo-2-thiophenecarboxylic acid (2.07 g, 10 mmol) in DMF (5 ml.) was added NCS (2.7 g, 15 mmol) in one portion. The reaction mixture was stirred at 50 0C for 10 h, and then cooled to room temperature. The desired product precipitated after water (5 ml.) was added. The white solid was filtered and dried under high vacuum to give 1.9 g (79%). LC-MS (ES) m/z =242 (M+H)+,
References:
SMITHKLINE BEECHAM CORPORATION WO2008/98104, 2008, A1 Location in patent:Page/Page column 177

24065-33-6
761 suppliers
$6.00/5g

60729-37-5
44 suppliers
$79.00/100mg
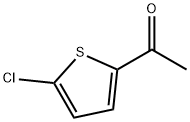
6310-09-4
210 suppliers
$9.00/5g

60729-37-5
44 suppliers
$79.00/100mg
123418-67-7
8 suppliers
inquiry

60729-37-5
44 suppliers
$79.00/100mg