Yield:-
Reaction Conditions:
with aluminum (III) chloride in dichloromethane at 0; for 5 h;Heating / reflux;
Steps:
2
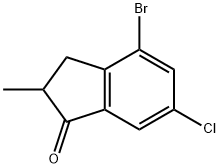
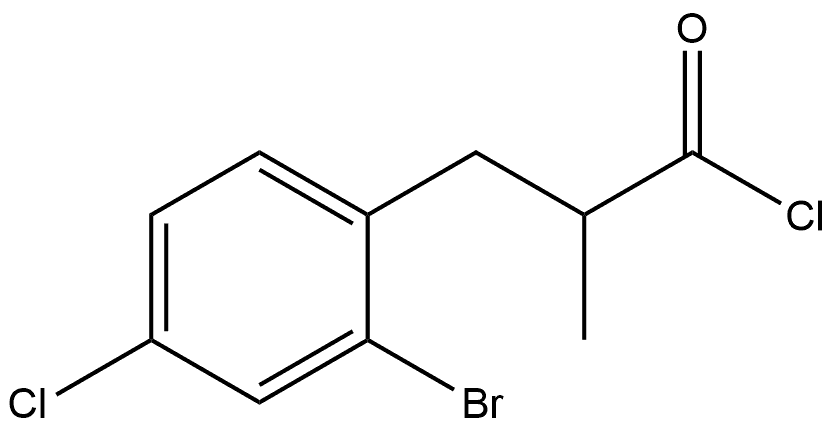
In a three-necked round-bottom 2000 ml flask equipped with a reflux condenser, dropping funnel with pressure-equalizing, and magnetic stirring bar, 20.5 g (0.87 mol) of sodium metal were dissolved in 500 ml of dry ethanol. To the resulting solution 152 g (0.87 mol) of diethylmethylmalonate in 150 ml of dry ethanol were added dropwise within 15 min. This mixture was stirred for 15 min; then, 252 g (0.89 mmol) of 2-bromo-4-chlorobenzyl bromide were added by vigorous stirring at a rate that allowed the reaction mixture to maintain a gentle reflux. Additionally, this mixture was refluxed for 4 h and cooled to room temperature. A solution of 173 g of KOH in 500 ml of water was added and the mixture was refluxed for 3 h to saponificate the ester formed. Ethanol and water were distilled off and 500 ml of water and, then, 12 M HCl (to pH 1) were added to the residue. The substituted methylmalonic acid that precipitated was separated, washed with 2 x 300 ml of cold water and dried in vacuum. Crude 3- (2-bromo-4-chlorophenyl)-2-methylpropanoic acid was obtained after decarboxylation of this substituted methylmalonic acid by heating it for 2.5 h at 190°C. The product was used without further purification. Mixture of this acid and 210 ml of SOCl2 was stirred for 24 h at ambient temperature. Thionyl chloride was distilled off. Fractional distillation gave 223 g of colorless oil of 3- (2-chlorophenyl)-2-methylpropanoyl chloride, b.p. 134-142°C/1 mm Hg. This acid chloride was dissolved in 200 ml of CH2Cl2 and was added dropwise by vigorous stirring to a suspension of 122 g (0.92 mol) Of AlCl3 in 750 ml Of CH2Cl2 for 2 h at 0°C. Then, this mixture was refluxed for 3 h, cooled to ambient temperature, and poured on 500 cm3 of ice. The organic layer was separated and the aqueous layer was extracted with 3 x 300 ml of methyl-/er/-butyl ether. The combined organic fractions were dried over K2CO3 and evaporated to dryness. To a solution of the resulting crude 4-bromo-6-chloro-2-methyl-l -indanone in 1000 ml of THF-methanol (2: 1, vol.) 42.0 g (1.1 1 mol) Of NaBH4 were added in small portions for 2 h at -50C (Caution: temperature must be lower O0C). The mixture was stirred for 12 h at ambient temperature. The resulting mixture was poured on 1000 cm3 of ice and acidified with 10% HCl to pH=4. The organic layer was separated and the aqueous layer was extracted with 3 x 250 ml of methyl-tert- butyl ether. This combined organic fractions was dried over K2CO3 and evaporated to dryness. To the residue 1500 ml of toluene were added and the resulting toluene solution was treated with a catalytic amount of ^ToISO3H (ca. 2 g) for 2 h at reflux. Then, the mixture was cooled to room temperature and passed through a short Silica Gel 60 column (40-63 μm, d 60 mm, 1 40 mm). This column was additionally eluted with 300 ml of toluene. The combined extract was evaporated to dryness. Fractional distillation gave a mixture of the title indenes, b.p. 115-121°C/2 mm Hg. Yield 144 g (67%) of colorless solid of ca. 1 to 5 mixture of 4-bromo-6-chloro-2-methylindene and 7-bromo-5-chloro-2- methylindene.Anal. calc. for C10H8BrCl: C, 49.32; H, 3.31. Found: C, 49.25; H, 3.30.1H NMR (CDCl3): 4-bromo-6-chloro-2-methylindene, δ 7.36 (m, IH, 7- H), 7.24 (m, IH, 5-H), 6.53 (m, IH, 3-H), 3.34 (m, 2H, 1,1 '-H), 2.16 (s, 3H, 2- Me); 7-bromo-5-chloro-2-methylindene, δ 7.23 (d, J= 1.6 Hz, IH, 6-H), 7.14 (d, J= 1.6 Hz, IH, 4-H), 6.44 (m, IH, 3-H), 3.23 (m, 2H, 1 ,1 '-H), 2.18 (s, 3H, 2-Me).
References:
WO2007/70041,2007,A1 Location in patent:Page/Page column 91-93