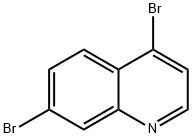
4-BROMO-7-BROMOQUINOLINE synthesis
- Product Name:4-BROMO-7-BROMOQUINOLINE
- CAS Number:700871-88-1
- Molecular formula:C9H5Br2N
- Molecular Weight:286.95

700871-86-9

700871-88-1
General procedure for the synthesis of 4,7-dibromoquinoline from compound (CAS: 700871-86-9): precursor compound 20 (3.15 g, 8.8 mmol) and lithium bromide (LiBr, 7.7 g, 88.5 mmol) were dissolved in 100 mL of acetonitrile (CH3CN). The reaction mixture was heated and stirred at 55 °C for 8 h under nitrogen protection. Upon completion of the reaction, the mixture was cooled to room temperature and concentrated under reduced pressure to a slurry. The crude product was diluted with ethyl acetate (EtOAc, 30 mL) and subsequently washed with saturated sodium bicarbonate (NaHCO3) solution. The organic phase was separated, extracted with saturated sodium chloride solution, dried over anhydrous sodium sulfate (Na2SO4), filtered and concentrated under reduced pressure to give the solid crude product. Purification by medium pressure liquid chromatography (MPLC) on a silica gel column with 25% ethyl acetate/hexane as eluent gave 1.9 g of white solid 4,7-dibromoquinoline in 75% yield. Mass Spectrometry (MS) ES+ m/e: 287.8 (M + 1).

700871-86-9
0 suppliers
inquiry

700871-88-1
58 suppliers
$88.00/250mg
Yield:700871-88-1 75%
Reaction Conditions:
with lithium bromide in acetonitrile at 55; for 8 h;under nitrogen;
Steps:
22 4,7-Dibromoquinoline
The product of Preparation 20 (3.15 g, 8.8 mmol) and LiBr (7.7 g, 88.5 mmol) are dissolved in 100 mL OF CH3CN and the mixture is heated at 55 °C for 8 h under nitrogen. The reaction mixture is cooled to RT and evaporated to a slurry. The crude mixture is diluted with EtOAc (30 mL) and washed with saturated NAHC03 solution. The organic phase is separated and extracted with saturated brine, dried (NA2S04), filtered, and evaporated to a solid mass. The crude product is purified by MPLC on silica gel (25% ETOAC/HEXANES) to yield the title compound, 1.9 g (75%), as white solid. MS ES+ m/e 287.8 (M+1).
References:
WO2004/48383,2004,A1 Location in patent:Page 51