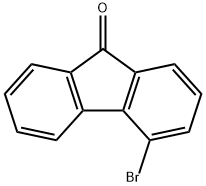
4-Bromo-9H-fluoren-9-one synthesis
- Product Name:4-Bromo-9H-fluoren-9-one
- CAS Number:4269-17-4
- Molecular formula:C13H7BrO
- Molecular Weight:259.1
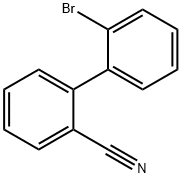
54245-41-9

4269-17-4
To a 500 mL three-necked flask were sequentially added 50 mL of benzyl cyanide, 70.7 g (0.3 mol) o-dibromobenzene and 68.4 g (0.45 mol) cesium fluoride. Under argon protection, 0.86 g (1.5 mmol) of bis(dibenzylideneacetone)palladium, 2.86 g (15 mmol) of thiophene-2-carboxylate, and 7.0 g (15 mmol) of 1,1'-bis(di-tert-butylphosphino)ferrocene were added as catalyst. The reaction mixture was stirred at 150°C for 24 hours. After completion of the reaction, it was cooled to room temperature, the reaction mixture was filtered and the filtrate was distilled to remove benzyl cyanide to give the crude product 2-bromo-2'-carbonitrile biphenyl. The crude product was transferred to another 500 mL three-necked flask and 80 mL of glacial acetic acid and 80 mL of concentrated sulfuric acid were added. After heating and refluxing for 12 hours, it was cooled to room temperature and filtered to obtain a dark yellow solid. The solid was washed with 100 mL of water and 100 mL of methanol and finally recrystallized from 200 mL of isopropylbenzene to give 61.9 g (80% yield) of 4-bromo-9H-fluoren-9-one as a yellow solid.

54245-41-9
3 suppliers
inquiry

4269-17-4
113 suppliers
$12.00/100mg
Yield:4269-17-4 80%
Reaction Conditions:
with sulfuric acid;acetic acid for 12 h;Reflux;
Steps:
3 Example 3
To a 500 mL reaction flask, 50 mL of benzonitrile was added successively,70.7 g (0.3 mol) of o-dibromobenzene,68.4 g (0.45 mol) of cesium fluoride,Catalyst purged with argon was added bis (dibenzylideneacetone) palladium 0.86g (15mmol),Thiophene-2-carboxylate 2.86 g (15 mmol)And 7.0 g (15 mmol) of 1,1'-bis (di-t-butylphosphine) ferrocene,150 reaction 24h,filter,The filtrate was distilled to remove benzonitrile to give crude 2-bromo-2'-carbonitrile biphenyl.A 500 mL reaction flask was charged with crude 2-bromo-2'-carbonitrile biphenyl,Glacial acetic acid 80mL,Concentrated sulfuric acid 80mL.After heating and refluxing for 12 h,Cooled to room temperature,filter,To give a dark yellow solid,100 mL of water,100 mL of methanol,Recrystallization from 200 mL of cumene afforded 61.9 g of 4-bromofluorenone as a yellow solid in 80% yield.
References:
CN105237379,2016,A Location in patent:Paragraph 0006; 0016
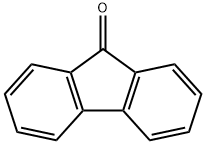
486-25-9
488 suppliers
$5.00/25g

4269-17-4
113 suppliers
$12.00/100mg

69200-16-4
11 suppliers
inquiry

4269-17-4
113 suppliers
$12.00/100mg
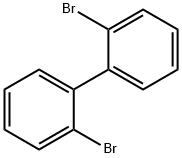
13029-09-9
286 suppliers
$20.00/1g

4269-17-4
113 suppliers
$12.00/100mg
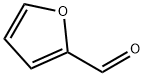
98-01-1
509 suppliers
$22.00/25g
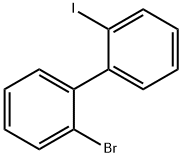
39655-12-4
45 suppliers
inquiry

486-25-9
488 suppliers
$5.00/25g

4269-17-4
113 suppliers
$12.00/100mg