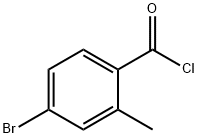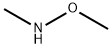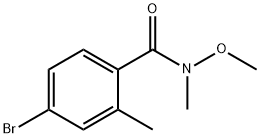
4-bromo-N-methoxy-N,2-dimethylbenzamide synthesis
- Product Name:4-bromo-N-methoxy-N,2-dimethylbenzamide
- CAS Number:178313-45-6
- Molecular formula:C10H12BrNO2
- Molecular Weight:258.11
Yield:178313-45-6 95%
Reaction Conditions:
with pyridine in dichloromethane;acetonitrile at 0 - 20; for 6.33333 h;
Steps:
Intermediate 8: (R)-2-(4-bromo-2-methylphenyl)propan-l-ol; Intermediate 8A:; [00211] To a suspension of 4-bromo-2-methylbenzoic acid (30.5 g, 142 mmol) in DCM (250 mL), were added oxalyl chloride (14.9 mL, 170 mmol) and DMF (20
References:
WO2008/79836,2008,A2 Location in patent:Page/Page column 93-94
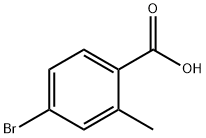
68837-59-2
349 suppliers
$5.00/1g

178313-45-6
9 suppliers
inquiry