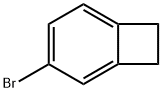
4-Bromobenzocyclobutene synthesis
- Product Name:4-Bromobenzocyclobutene
- CAS Number:1073-39-8
- Molecular formula:C8H7Br
- Molecular Weight:183.05
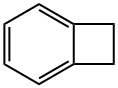
694-87-1

1073-39-8
The general procedure for the synthesis of 4-bromobenzocyclobutene from benzocyclobutene is as follows: first, α-chloro-o-xylene 1 is subjected to a pyrolysis reaction at about 800 °C and 0.5 mbar to produce benzocyclobutene 2 in 45% yield. Subsequently, benzocyclobutene 2 was dissolved in acetic acid and reacted with a mixture of bromine and iodine at room temperature for selective bromination to give 4-bromobenzocyclobutene 3. 3 was dissolved in toluene and a slightly molar excess of 1,4-dihydro-1,4-epoxynaphthalene 4 was added and reacted for 20 hr at 220° C. to give the Diels-Alder addition product 5 as a pure inner/outer in 80% yield mixture, which was a colorless crystalline substance. -yl)-5,12-dihydroanthracene 7. After recrystallization of compound 7 with o-dichlorobenzene, the dehydrogenation reaction was carried out by treatment with 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ) in boiling o-xylene. After repeated vacuum sublimation purification, orange-red crystals of 2-(anthracen-2-yl)tetraphenyl 8 were finally obtained in 75% yield. All intermediates were characterized by 1H NMR, 13C NMR spectroscopy and mass spectrometry. Compound 8 was characterized by UV-visible spectroscopy.

694-87-1
168 suppliers
$22.00/250mg

1073-39-8
189 suppliers
$14.00/250mg
Yield:1073-39-8 76.9%
Reaction Conditions:
with bromine in water at 20; for 4.5 h;Cooling with ice;Darkness;Reagent/catalyst;
Steps:
1-2 Preparation of 4-bromobenzocyclobutene
Add 2g (20mmol) of benzocyclobutene to a 50mL three-necked flask, then add 12mL of water solvent, transfer to a stirring table placed in an ice bath and stir (450r/min) in the dark. After starting to stir, use A constant pressure dropping funnel was slowly dropped into 1.2 mL (20 mmol) of liquid bromine, the ice bath was removed after the bromine was dropped, and the reaction was stirred for 4.5 hours in the dark at room temperature. After stopping the reaction, extract the reaction mixture with n-hexane, adjust pH=7, collect the organic phase and remove the organic solvent by rotary evaporation, and then separate the pure monobromo target product (4Br-BCB) obtained by vacuum distillation . The substance is a colorless liquid, the selective yield of monobromine substitution is 76.9%, and the yield of polybrominated substitution is 21.5%.
References:
CN111908998,2020,A Location in patent:Paragraph 0043-0054
![Bicyclo[4.2.0]octa-1,3,5-triene, 3-bromo-7,8-diiodo-](/CAS/20210305/GIF/4181-31-1.gif)
4181-31-1
0 suppliers
inquiry

1073-39-8
189 suppliers
$14.00/250mg
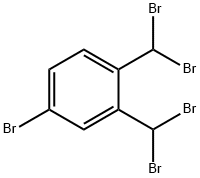
4235-46-5
34 suppliers
$45.00/50mg

1073-39-8
189 suppliers
$14.00/250mg
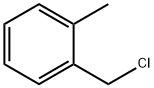
552-45-4
344 suppliers
$21.00/25g

1073-39-8
189 suppliers
$14.00/250mg