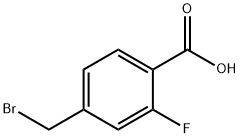
4-(Bromomethyl)-2-fluorobenzoicacid synthesis
- Product Name:4-(Bromomethyl)-2-fluorobenzoicacid
- CAS Number:477199-77-2
- Molecular formula:C8H6BrFO2
- Molecular Weight:233.03
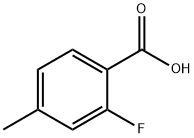
7697-23-6
243 suppliers
$7.00/1g

477199-77-2
12 suppliers
$182.00/250mg
Yield:477199-77-2 62%
Reaction Conditions:
with N-Bromosuccinimide;2,2'-azobis(isobutyronitrile) in tetrachloromethane at 90; for 3 h;
Steps:
13.1
[00202] Step 1 : AIBN (533 mg, 3.24 mmol) and N-bromosuccinimide (6.35 g, 35.68 mmol) were added to 2-fluoro-4-methyl-benzoic acid (5.0 g, 32.4 mmol) in CC14 (50 mL) and the reaction mixture heated for 3 h at 90 °C. The reaction mixture was cooled, filtered, and the filter cake was washed once with CC14, then three times with water. The filter cake was then dissolved in a 1 : 1 mixture of acetonitrile/MeOH, dried over a2S04, filtered and concentrated in vacuo to afford 4-(bromomethyl)-2-fluoro-benzoic acid (4.7 g, 62% yield) as an off-white solid. ? NMR (400 MHz, DMSO-i?) 513.30 (s, 1H), 7.89 - 7.81 (m, 1H), 7.42 (dd, J = 8.9, 4.0 Hz, 1H), 7.38 (dd, J = 8.0, 1.5 Hz, 1H), 4.73 (s, 2H).
References:
WO2011/143426,2011,A1 Location in patent:Page/Page column 71
214554-18-4
41 suppliers
$42.00/250mg

477199-77-2
12 suppliers
$182.00/250mg
452-74-4
303 suppliers
$17.00/25g

477199-77-2
12 suppliers
$182.00/250mg
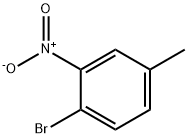
5326-34-1
255 suppliers
$6.00/5g

477199-77-2
12 suppliers
$182.00/250mg
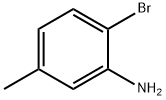
53078-85-6
198 suppliers
$6.00/1g

477199-77-2
12 suppliers
$182.00/250mg