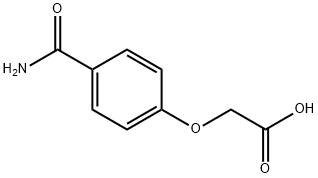
(4-Carbamoylphenoxy)acetic Acid synthesis
- Product Name:(4-Carbamoylphenoxy)acetic Acid
- CAS Number:29936-86-5
- Molecular formula:C9H9NO4
- Molecular Weight:195.17
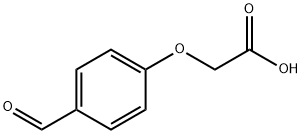
22042-71-3
127 suppliers
$14.00/1g

29936-86-5
18 suppliers
inquiry
Yield:29936-86-5 80%
Reaction Conditions:
Stage #1: (4-formylphenoxy)acetic acidwith hydroxylamine hydrochloride in formic acid; for 2 h;Reflux;
Stage #2: with potassium hydroxide in tert-butyl alcohol; for 4 h;Reflux;
Steps:
4 INTERMEDIATE 4 2-(4-Carbamoylphenoxy)acetic acid
INTERMEDIATE 42-(4-Carbamoylphenoxy)acetic acidTo a solution of Intermediate 3 (1.9 g, 10.3 mmol) in formic acid (50 mL) wasadded hydroxylamine hydrochloride (1 .1 g, 15.5 mmol). The reaction mixture was heatedat reflux for 2 h, then cooled and concentrated in vacuo. The residue was triturated with isohexane and filtered. The resulting cream solid was suspended in tert-butanol (50 mL), then potassium hydroxide (2.3 g, 41.0 mmol) was added and the reaction mixture was heated at reflux for 4 h. The reaction mixture was cooled and water (100 mL) was added.The reaction mixture was partitioned with EtOAc (2 x 100 mL), and the combined extracted aqueous layer was adjusted to pH 2 with 6N HC1. The resulting precipitate was filtered off and dried in vacuo, yielding the title compound (1.6 g, 80%) as a white solid. H (d6-DMSO) 7.84-7.8 1 (m, 3H), 7.17 (br s, 1H), 6.96-6.94 (m, 2H), 4.74 (s, 2H).LCMS (ESj 196.0 (M+H), RT 0.30 minutes (Method B).
References:
WO2015/86509,2015,A1 Location in patent:Page/Page column 93
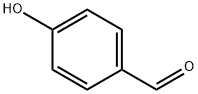
123-08-0
870 suppliers
$5.00/10g

29936-86-5
18 suppliers
inquiry