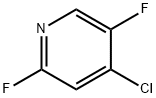
4-Chloro-2,5-difluoropyridine synthesis
- Product Name:4-Chloro-2,5-difluoropyridine
- CAS Number:851386-40-8
- Molecular formula:C5H2ClF2N
- Molecular Weight:149.53
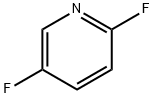
84476-99-3

851386-40-8
General procedure for the synthesis of 4-chloro-2,5-difluoropyridine using 2,5-difluoropyridine as starting material: diisopropylamine (53 g, 0.525 mol) was added to a solvent mixture of tetrahydrofuran (150 ml) and methyl tert-butyl ether (200 ml) under nitrogen protection. The reaction system was cooled to -60 to -40 °C and a hexane solution of n-butyllithium (191 ml, 2.5 M) was slowly added. Subsequently, the reaction temperature was slowly raised to -20°C and stirred at this temperature for 10 minutes. The reaction flask was again cooled to -75 °C and a solution of 2,5-difluoropyridine (50 g, 0.434 mol) was slowly added dropwise for a controlled time of 1 hour. After the dropwise addition was completed, the addition of Freon-113 (89.4 g, 0.478 mol) solution was continued dropwise at -75 °C. After completion of dropwise addition, the reaction system was kept insulated at -75°C for 2 hours. At the end of the reaction, the reaction was quenched with saturated ammonium chloride solution and then the reaction solution was extracted with methyl tert-butyl ether. The organic phase was washed sequentially with 2N aqueous hydrochloric acid solution, water, saturated sodium bicarbonate solution and saturated brine. The organic phase was separated, dried with anhydrous sodium sulfate and filtered. The filtrate was first distilled at 80 °C under atmospheric pressure to remove the low-boiling solvent, then distilled at 120 °C under reduced pressure, and finally distilled at 90 °C under atmospheric pressure to obtain the target product 4-chloro-2,5-difluoropyridine (50 g, 78% yield).

84476-99-3
280 suppliers
$8.00/1g

851386-40-8
48 suppliers
$46.00/100mg
Yield: 78%
Reaction Conditions:
Stage #1:2,5-difluoropyridine with n-butyllithium;diisopropylamine in tetrahydrofuran;hexane;tert-butyl methyl ether at -78; for 1 h;
Stage #2: with 1,1,2-Trichloro-1,2,2-trifluoroethane in tetrahydrofuran;hexane;tert-butyl methyl ether at -75; for 2 h;Heating;
Steps:
1.1; 2.1; 3.1 Synthesis of 4-chloro-2,5-difluoropyridine:
Diisopropylamine (53 g, 0.525 mol) was added to tetrahydrofuran (150 ml) / methyl tert-butyl ether (200 ml)Of the mixed solution,Replace nitrogen.Cooling to -60 - 40 ,A solution of n-butyllithium / n-hexane (191 ml, 2.5 M)The temperature was then slowly warmed to -20 ° C and stirred for 10 minutes.The reaction flask was cooled to -75 ° C,A solution of 2,5-difluoropyridine (50 g, 0.434 mol)After dripping for 1 hour.A solution of Freon-113 (89.4 g, 0.478 mol) was added dropwise at -75 ° C,Dripping finished insulation for 2 hours.Saturated ammonium chloride quenching reaction,Methyl tert-butyl ether extraction reaction solution.The organic phase was washed with 2N aqueous hydrochloric acid,And then washed with water,Saturated sodium bicarbonate wash,Saturated brine washing,The organic phase was separated and dried over anhydrous sodium sulfate,filter.The filtrate was distilled at 80 ° C to 120 ° C under normal pressure to remove the solvent,The residue was distilled at 120 ° C to 190 ° C under atmospheric pressure to give the product 4-chloro-2,5-difluoropyridine (50 g, yield 78%).
References:
Danuo Pharmaceutical (Suzhou) Co., Ltd.;Ding, Jun;He, Shijie;Zhuang, Zhijun;Ma, Zhenkun CN106432222, 2017, A Location in patent:Paragraph 0038; 0039; 0040-0042; 0060-0064; 0079-0083