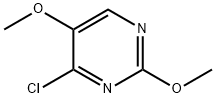
4-chloro-2,5-diMethoxypyriMidine synthesis
- Product Name:4-chloro-2,5-diMethoxypyriMidine
- CAS Number:370103-25-6
- Molecular formula:C6H7ClN2O2
- Molecular Weight:174.59
370103-23-4
58 suppliers
$8.00/10g

370103-25-6
67 suppliers
$99.00/100mg
Yield: 72%
Reaction Conditions:
with sodium hydroxide;triethylamine;trichlorophosphate in ice-water;toluene
Steps:
4 Example 4
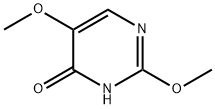
Example 4 15.6 g (0.1 mol) of 2,5-dimethoxy-4-hydroxypyrimidine from Example 3 were suspended in 25 g of toluene. 46.0 g of phosphorus oxychloride were added and the suspension heated to 80° C. Within 1 hour, 20.2 g of triethylamine were added dropwise and the entire mixture was stirred at 80° C. for 30 minutes. The entire mixture was poured into 600 g of ice-water, stirred for 12 hours and adjusted to pH 5 using sodium hydroxide. The toluene phase was removed, and the aqueous phase extracted a further 3 times with 50 mol of toluene each time. The combined toluene extracts were concentrated to dryness. 12.57 g of 4-chloro-2,5-dimethoxypyrimidine were obtained in the form of slightly yellowish crystals of characteristic odor. Melting point: 78 to 80° C., 1H-NMR: 3.860 ppm (s, 3H), 3.909 ppm (s, 3H), 8.446 ppm (s, 1H), 13C-NMR: 55.11 ppm (OCH3), 57.42 ppm (OCH3), 143.90 ppm (C6), 145.09 ppm (C5), 149.67 ppm (C4), 158.28 ppm (C2). GC/MS data: content>98%, M+ 174/176 g/mol. The yield was 72%.
References:
Güthner, Thomas;Neuhauser, Karl-Heinz US2003/22908, 2003, A1