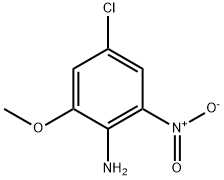
4-chloro-2-methoxy-6-nitroaniline synthesis
- Product Name:4-chloro-2-methoxy-6-nitroaniline
- CAS Number:859877-49-9
- Molecular formula:C7H7ClN2O3
- Molecular Weight:202.6
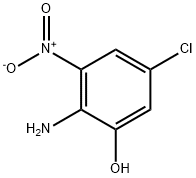
924274-94-2

74-88-4

859877-49-9
Step 2. Synthesis of 4-chloro-2-methoxy-6-nitroaniline. In a dry reaction flask, 2-amino-5-chloro-3-nitrophenol (752 mg, 4.0 mmol) and potassium carbonate (415 mg, 3.0 mmol) were dissolved in N,N-dimethylformamide (DMF). To the above mixture, iodomethane (625 mg, 4.4 mmol) was slowly added at room temperature. The reaction mixture was stirred continuously for 2 hours at room temperature. After completion of the reaction, the reaction was quenched with water and extracted with ethyl acetate (EtOAc). The organic layers were combined, washed with saturated brine and dried over anhydrous sodium sulfate. The solvent was removed by concentration under reduced pressure to give the crude product. The crude product was purified by fast column chromatography to afford the target compound 4-chloro-2-methoxy-6-nitroaniline (450 mg, 46.4% yield). The product was analyzed by LC-MS and showed m/z 203 ([M-H]+).
Yield:859877-49-9 46.4%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 20; for 2 h;
Steps:
20.2 Step 2. 4-Chloro-2-methoxy-6-nitroaniline.
Step 2. 4-Chloro-2-methoxy-6-nitroaniline. To a mixture of 2-amino-5- chloro-3-nitrophenol (752 mg, 4.0 mmol) and potassium carbonate (415 mg, 3.0 mmol) in DMF was added Mel (625 mg, 4.4 mmol). The reaction mixture was stirred at r.t. for 2hr, then quenched with water and extracted with EtOAc. Combined organic layers were washed with brine, dried over anhydrous Na2S04 and concentrated under reduced pressure. The residue was purified by flash column chromatography to give 4-chloro-2- methoxy- 6-nitroaniline (450 mg, 46.4% yield). LC-MS: m/z 203(M-H)+.
References:
WO2014/176258,2014,A1 Location in patent:Page/Page column 65
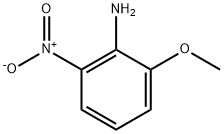
16554-45-3
140 suppliers
$16.00/1g

859877-49-9
26 suppliers
inquiry
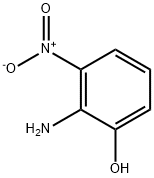
603-85-0
271 suppliers
$15.00/5g

859877-49-9
26 suppliers
inquiry