
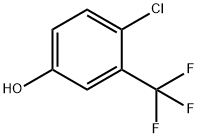
4-Chloro-2-nitro-5-trifluoromethyl-phenol synthesis
- Product Name:4-Chloro-2-nitro-5-trifluoromethyl-phenol
- CAS Number:1192021-48-9
- Molecular formula:C7H3ClF3NO3
- Molecular Weight:241.55
Yield:1192021-50-3 26% ,1192021-48-9 40%
Reaction Conditions:
with sulfuric acid;nitric acid;acetic acid at 20; for 3 h;
Steps:
26.a a) A: 2-Chloro-5-hydroxy-4-nitrobenzotrifluoride, B: 2-Chloro-5-hydroxy-4-nitrobenzotrifluoride
Under ice cooling, sulfuric acid (3 mL) was added to a solution of 2-chloro-5-hydroxybenzotrifluoride (5 g, 25.4 mmol) in acetic acid (20 mL). 69% Nitric acid (2 mL) was slowly added dropwise to the reaction mixture, and the resulting mixture was warmed to room temperature, and stirred for 3 hours. The reaction mixture was poured into ice water, the resulting mixture was neutralized with sodium hydrogencarbonate, and then ethyl acetate was added to the reaction mixture. The organic layer was washed 3 times with water, then washed with saturated aqueous sodium chloride, dried over anhydrous magnesium sulfate, and filtered, and then the solvent was evaporated under reduced pressure. The resulting residue was purified by flash column chromatography, and dried to obtain crystals of the title compound. A (1.6 g, yield 26%) and crystals of the title compound B (2.5g, yield 40%).A: 2-Chloro-5-hydroxy-4-nitrobenzotrifluoride 1H-NMR (CDCl3) δ: 7.57 (1H, s), 8.27 (1H, s), 10.43 (1H, s) B: 2-Chloro-5-hydroxy-4-nitrobenzotrifluoride1H-NMR (CDCl3) δ: 7.23 (1H, d, J=8.9 Hz), 7.52 (1H, d, J=9.2 Hz)
References:
US2017/298081,2017,A1 Location in patent:Paragraph 0288-0293
6294-93-5
166 suppliers
$6.00/1g

1192021-48-9
4 suppliers
inquiry
