
4-chloro-3-(1-isoquinolinyl)Benzenamine synthesis
- Product Name:4-chloro-3-(1-isoquinolinyl)Benzenamine
- CAS Number:1258282-48-2
- Molecular formula:C15H11ClN2
- Molecular Weight:254.71

1258282-47-1
0 suppliers
inquiry

1258282-48-2
3 suppliers
inquiry
Yield:1258282-48-2 92%
Reaction Conditions:
Stage #1: 1-(2-chloro-5-nitrophenyl)isoquinolinewith ethanol;tin(ll) chloride at 70; for 1.5 h;
Stage #2: with sodium hydrogencarbonate in water;Cooling with ice;
Steps:
3
Example 3A mixture of 2 1-(2-chloro-5-nitrophenyl)isoquinoline (785 mg, 2.76 mmol) and tin (II) chloride dehydrate (2.93 g, 12.97 mmol) in ethanol (36.8 ml_) was heated at 700C for 1.5 hr. The reaction mixture was cooled down and then poured into ice-water followed by neutralization with saturated NaHCO3 solution. The mixture was filtered through a pad of celite and washed with EtOAc. The filtrate was extracted with EtOAc and the combined extracts were washed with brine, dried over anhydrous Na2SO4 and then concentrated under reduced pressure to yield the desired compound (650 mg, 92%) as yellow solid. 1 H NMR (400 MHz, CDCI3): δ 8.61 (d, J = 5.6 Hz, 1 H), 7.88 (d, J = 8.0 Hz, 1 H), 7.75-7.67 (m, 3H), 7.53 (m, 1 H), 7.29 (dd, J = 0.8, 8.0 Hz, 1 H), 6.76 (m, 2H), 3.76 (br s, 2H). MS (ESI): Calcd. for C15H12CIN2: 255, found 255 (M+H)+.
References:
WO2010/144586,2010,A1 Location in patent:Page/Page column 81
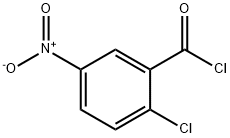
25784-91-2
159 suppliers
$39.00/5G

1258282-48-2
3 suppliers
inquiry
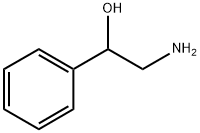
7568-93-6
200 suppliers
$13.43/500mgs:

1258282-48-2
3 suppliers
inquiry