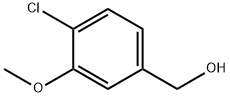
(4-Chloro-3-Methoxyphenyl)Methanol synthesis
- Product Name:(4-Chloro-3-Methoxyphenyl)Methanol
- CAS Number:13726-17-5
- Molecular formula:C8H9ClO2
- Molecular Weight:172.61
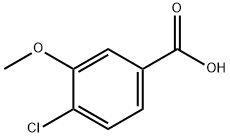
85740-98-3

13726-17-5
Step 1. Dissolve 4-chloro-3-methoxybenzoic acid (1.12 g, 5.98 mmol) in tetrahydrofuran (30 mL) under ice bath conditions with stirring. A tetrahydrofuran solution of 1.0 M lithium aluminum hydride (7.18 mL, 7.18 mmol) was slowly added over approximately 5 minutes. Ice bath conditions were maintained and stirring of the reaction mixture was continued for 2 hours. Upon completion of the reaction, the reaction was quenched by slow dropwise addition of water (0.27 mL), followed by addition of 15% aqueous sodium hydroxide solution (0.27 mL), and finally water (0.82 mL). The resulting suspension was filtered and the collected solid was washed with ethyl acetate. The filtrates were combined and concentrated under reduced pressure to give (4-chloro-3-methoxyphenyl)methanol as a colorless oil (737 mg, 71% yield). The product was characterized by 1H NMR (400 MHz, CDCl3): δ 3.92 (s, 3H), 4.67 (s, 2H), 6.87 (d, J = 7.6 Hz, 1H), 6.98 (s, 1H), 7.33 (d, J = 7.6 Hz, 1H).

85740-98-3
166 suppliers
$9.00/250mg

13726-17-5
59 suppliers
$45.00/2.5g
Yield:13726-17-5 99%
Reaction Conditions:
in tetrahydrofuran;
Steps:
C C.
C. Synthesis of (4-chloro-3-methoxyphenyl)methan-1-ol To a solution of 4-chloro-3-methoxybenzoic acid (4.88 g, 26.2 mmol) in THF (50 mL) was added borane-THF complex (52 mL 1M solution in THF, 52 mmol) via addition funnel over 10 min. The reaction mixture was refluxed for 2 hr, cooled, extracted with EtOAc, washed with water, 5% Na2CO3 and brine, dried and concentrated in vacuo to give (4-chloro-3-methoxyphenyl)methan-1-ol (4.5 g, 99%). ES-MS (M+H-H2O)+=155, 157 (Cl).
References:
US2002/77486,2002,A1
13726-16-4
97 suppliers
$8.00/1g

13726-17-5
59 suppliers
$45.00/2.5g

116022-18-5
69 suppliers
$50.00/25g

13726-17-5
59 suppliers
$45.00/2.5g
73909-16-7
52 suppliers
$50.00/1g

13726-17-5
59 suppliers
$45.00/2.5g
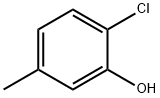
615-74-7
119 suppliers
$16.00/5g

13726-17-5
59 suppliers
$45.00/2.5g